Hepatitis C in Children
Hepatitis C incidence, transmission and risk factors
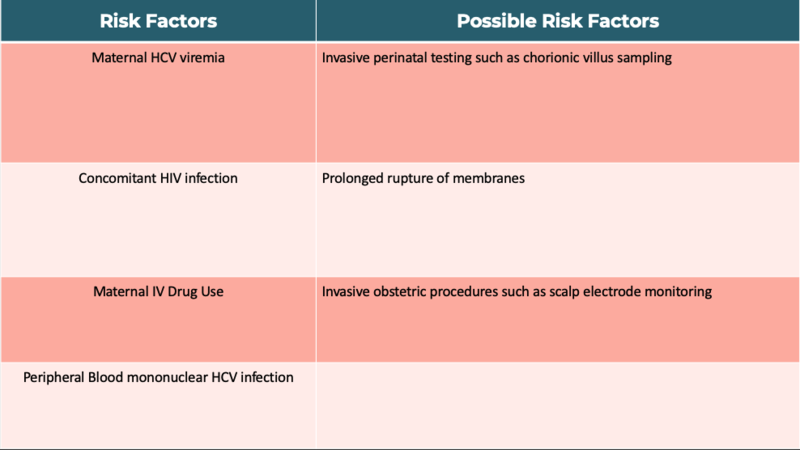
Hepatitis C has become the most common cause of chronic viral hepatitis in children in industrialized nations. Transmission can occur via blood transfusion, IV drug use, tattoos, sexual intercourse, and rarely solid-organ transplantation, however the most common source of transmission in children is mother to child in utero or during the peripartum period. The rate of transmission is approximately five percent but may be higher in those with a higher viral load and in those women that are HCV/HIV coinfected. Although transmission is higher in those with a higher HCV load, there is no exact HCV RNA level that precludes vertical transmission. Furthermore, transmission has also been reported in mothers’ that did not have a detectable viral load, thus a negative hepatitis C RNA quantitative PCR does not preclude the possibility of transmission. Factors that increase vertical transmission include maternal IV drug use during pregnancy and HCV peripheral blood mononuclear infection, with one theory proposing that PBMCs serve as a vector for HCV. Other proposed risk factors that may increase transmission include invasive prenatal testing such as chorionic villus sampling, prolonged rupture of membranes and invasive obstetric procedures such as scalp electrode monitoring.
Table 1: Perinatal Acquisition Risk Factors for Hepatitis C
Natural History and Who to Test
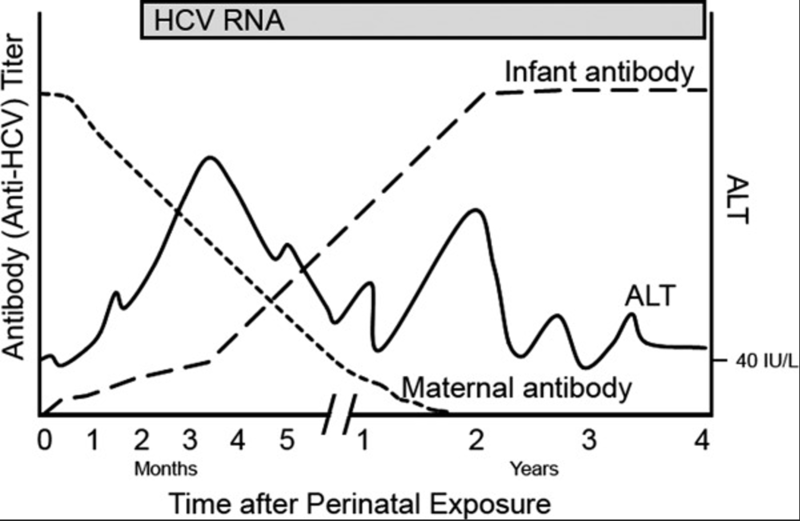
Approximately 25-40% of children who have contracted hepatitis C in the perinatal period may spontaneously clear the infection, typically by two years of age. Those with higher ALT levels are more likely to spontaneously clear the virus. After the age of two, the chance of spontaneous clearance of hepatitis C prior to age 19 decreases to 6-12%. If the virus is not cleared there is potential for progressive liver disease, however the onset is typically insidious and typically advanced liver disease does not occur until children reach adulthood. However, rarely, decompensated cirrhosis and hepatocellular carcinoma (HCC) can occur in pediatric patients.
Given the risk for development of advanced liver disease, the American Academy of Pediatrics recommends screening children whose mothers were infected with HCV during pregnancy, have a history of IV drug use, or are maternal siblings of children with vertically acquired HCV. Initial testing recommendation begins with an anti-HCV antibody at 18 months of age. Any patient >18 months with a positive anti-HCV antibody test should then be evaluated for active HCV-infection with an HCV RNA PCR test. The rational to delay HCV antibody testing until 18 months of age is to avoid earlier detection of transplacentally acquired maternal HCV-Ab as maternal antibodies (and therefore anti-HCV antibodies) may circulate in children for up to 18 months. In addition, as discussed above, infants often spontaneously clear HCV infection so delaying testing may allow for more spontaneous clearance. However, if there is concern for follow up, an alternative testing strategy can include obtaining HCV RNA PCR starting at two months of age and continuing to follow with repeated testing given the chance of spontaneous clearance. If a child is found to have hepatitis C infection, any siblings with the same mother should also be screened if they were not previously screened.
Figure 1 Pattern of Events in Children Following Perinatal HCV infection
Genotypes
Hepatitis C is a single stranded RNA protein with at least six genotypes which typically vary by region. Genotype 1 and 2 are most prevalent in Western countries, 3 in South and East Asia but can also be seen in the US, type 4 in North Africa and the Middle East, 5 in South Africa and 6 in Asia. Previously genotyping was an important component on directing Hepatitis C therapy. However, as discussed below most treatment regimens are now effective against all genotypes. However, data suggests that genotype 3 has a higher rate of spontaneous viral clearance.
Monitoring
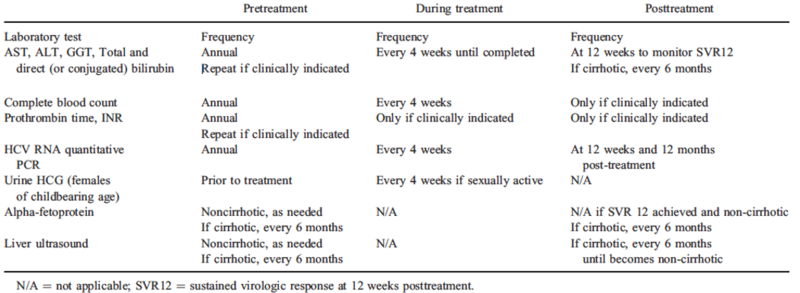
Serum AST/ALT levels can fluctuate in those with chronic Hepatitis C and studies have demonstrated that pediatric patients can have normal AST/ALT despite histologic evidence of infection. Once HCV RNA PCR is positive, if not receiving antiviral therapy, children should have semiannual to annual labs including HCV RNA level, CBC, AST, ALT, bilirubin, albumin, INR and urinalysis. Typically, abdominal US and AFPs are not indicated unless there is a family history of rapid disease progression to hepatocellular carcinoma or a personal history of cirrhosis or coinfection of hepatitis B, in which case AFP and abdominal US should be completed every six months. In addition, liver biopsies are rarely needed to direct next steps.
Clinical Manifestations of Hepatitis C Infection
Acute liver failure from hepatitis C in children is uncommon and those with chronic hepatitis C usually do not exhibit symptoms. However, some patients do present with fatigue, abdominal pain, jaundice, or hepatomegaly.Though rare, there have been case reports of membranoproliferative glomerulonephritis in children as well as thyroid autoimmune disease.A large multicenter prospective study in Europe followed 262 children with vertical HCV infection until 4.2 years of age and found only hepatomegaly as a reported symptoms in 10% if children which was associated with higher ALT values. Given the mostly asymptomatic nature of disease and the potential to cause fibrosis and cirrhosis, screening pregnant women and those children who are at risk for HCV as detailed above is essential.
Treatment
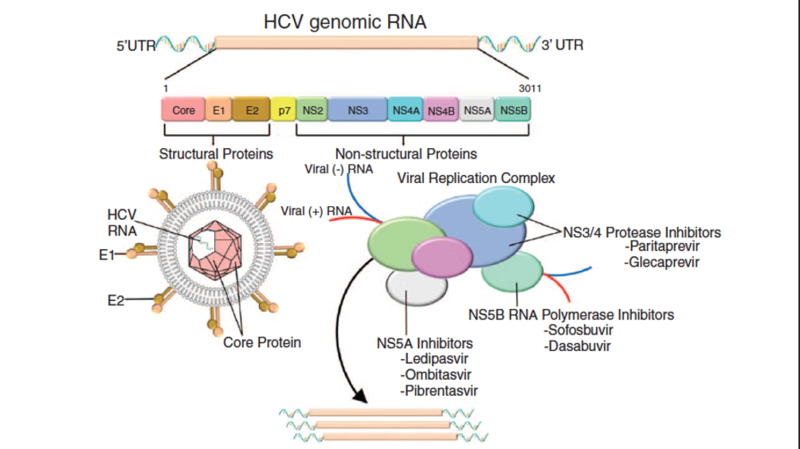
Historically, treatment was strongly dictated by HCV genotype, however with the FDA approval of highly effective, pan-genotypic oral direct-acting antivirals (DAA) for pediatric patients, HCV treatment has changed significantly. Because reactivation of HBV has been reported in those with HCV and HBV infection while taking DAA, testing for hepatitis B infection should be completed before initiating therapy for HCV. In addition, the AASLD does not recommend treatment for children under three years of age given there are no DAA’s approved for this age group, the chance of spontaneous clearance is high < 3 years of age, and the risk of significant liver disease due to HCV <3 years of life is very low. There are currently three options available to pediatric patients three years and older. Sofosbuvir-velpatasvir(Epclusa), glecaprevir-pibrentasvir(Mavyret) and ledipasvir-sofosbuvir (Harvoni). Medications are available in granules however they should still be swallowed without chewing. During treatment laboratory work including CBC, AST, ALT, GGT total and direct bilirubin, INR and HCV RNA should be checked every four weeks. Figure two displays the targets of DAA therapy.
Figure 2: HCV DNA mechanism of action
Long Term Monitoring of Hepatitis C Infection
Twelve weeks after completion of therapy as well as one year post completion of therapy, HCV RNA PCR should be checked. If the HCV RNA is undetectable, no long term follow up is needed unless there is a history of cirrhosis. Table 2 lists the pretreatment, during treatment and post treatment monitoring for HCV infection.