Malignant Transformation of Hepatocellular Adenomas
Introduction
The incidence of HCA was estimated to be 3-4 per 100,000 in females who use oral contraceptive pills (OCP) vs 0.13 per 100,000 in those without OCP use, based on a case-control study in 1979. Since then, per review of case reports and case series it appears that the incidence has increased, particularly in men, and possibly in association with an increase in the prevalence of obesity. However, true incidence is difficult to determine given potential bias in case reports and case series, as well as the fact that many HCAs are asymptomatic and may be undiagnosed. HCAs are also associated with glycogen storage disease (GSD) and androgen treatments, but these conditions account for less than 5% of cases.
Types of HCA
HCAs are the result of a monoclonal benign proliferation of hepatocytes. Bioulac-Sage et al described 4 main subtypes of HCA based on genotypic classification; HNF1A inactivated (H-HCA), inflammatory HCA (I-HCA), β-catenin activated HCA (b-HCA), and unclassified HCA. H-HCA account for 30-40% of adenomas and are characterized by activation of lipogenesis. They are almost exclusively found in women and can be associated with mature onset of diabetes of the young (MODY) type 3. HNF1A inactivated HCAs are not associated with increased risk of malignant transformation. I-HCAs are the most common, comprising 40-50% of adenomas. They are characterized by activation of STAT3, a transcription factor of inflammation. They are associated with obesity, alcohol intake, and oral contraceptive pill (OCP) use, and are also not associated with increased risk of malignant transformation. B-HCAs are characterized by a mutation or deletion at exon 3 of CTNNB1, which leads to activation of WNT/beta-catenin pathway. They are more common in males, as well as those on androgen treatment and in patients with GSD. These HCAs are associated with a higher rate of malignant transformation; 20-30% of HCAs that undergo malignant transformation have β-catenin transformation. A mutation at exon 7 or 8 of CTNNB1 has also been identified, which leads to faint beta-catenin activation, and is not associated with risk of malignant transformation. Of note, about 10% of I-HCA also have been found to have β-catenin mutations and are also associated with increased risk of malignant transformation. Recently sonic hedgehog activated HCA has been described as a new subtype, comprising 5% of HCAs. They are characterized by fusion of inhibin beta E subunit (INHBE) and GLI1 genes, leading to activation of the sonic hedgehog pathway.
Table 1: HCA Subtypes and Characteristics
| HCA subtype | Characteristics | Malignant potential |
| HNF1A inactivated |
|
No increased risk of malignant transformation |
| β-catenin activated |
|
Increased risk of malignancy
|
| Inflammatory |
|
No increased risk of malignant transformation
|
| Sonic hedgehog |
|
No increased risk of malignant transformation |
| Unclassified |
|
Unknown malignant risk |
Malignant transformation
Malignant transformation has been reported to occur in 4-8% of HCAs. This may be an over-estimation as it is based at least in part on studies with surgical resection cohorts. A systematic review of 1600 adenomas from 2010 reported frequency of malignant transformation to be 4.2%. HCCs that arise from HCAs are less likely to exhibit vascular involvement or satellite lesions, and are usually well-differentiated tumors. While rising AFP may be associated with malignant transformation, these HCCs often present with normal AFP.
Several risk factors have been associated with increased risk of malignant transformation in patients with HCAs (Table 2). Male sex is a major risk factor for malignant transformation. Males are less likely to have HCAs, however are more likely to have b-HCA and are reported to have 6-10 times the risk of malignant transformation compared to females. Cohort studies report up to 47% of males with HCAs develop HCC, compared to 4% of females. Size of HCA is also associated with risk of malignant transformation, with lesions greater than 5 cm considered to be high risk. In a systematic review of 1600 HCAs, only 3 cases (4.4%) of HCC occurred in lesions less than 5 cm. In contrast, number of HCA lesions is not associated with risk of malignant transformation. History of Type I GSD is also associated with increased risk of malignant transformation.
Table 2: Risk Factors for Malignant Transformation of HCA
| Male sex |
| β-catenin activated HCA |
| HCA size > 5 cm |
| Increased or rising AFP |
| Type I GSD |
Back to the case:
The patient is at increased risk of malignant transformation given male sex. He is also more likely to have a b-HCA. Number of HCA lesions is not associated with risk of HCC. He has a normal AFP, though this is not uncommon in patients with malignant transformation of HCA.
Diagnosis
Most HCAs are diagnosed by imaging. US, CT, and MRI have been studied for the purpose of detecting HCA. MRI is considered the most comprehensive imaging modality for HCAs and studies have shown that MRI can accurately identify two HCA subtypes, HNF-1α inactivated as well as inflammatory HCAs. Studies have also reported characteristic MRI findings for β-catenin HCA, however guidelines differ on these findings and whether β-catenin HCA can be identified based on MRI alone. EASL 2016 guidelines characterize β-catenin HCA as heterogeneously hyperintense on T2-weighted sequences, hypointense on T1-weighted sequences, and central scar but no signal loss on chemical shift sequences. EASL does not recommend diagnosis of β-catenin subtype based on imaging alone. ACG 2014 guidelines describe b-HCA as heterogeneous with no signal dropout, isointense on T1 and T2 with strong arterial enhancement and delayed washout. The inflammatory b-HCA subtype is noted to have same appearance as I-HCA. ACG states that imaging can be sufficient for identification of different subtypes. Both guidelines agree that biopsy is not routinely recommended for HCA diagnosis, but rather only in cases where diagnosis is uncertain or when there is concern for malignancy. Further studies examining imaging characteristics is warranted, especially given the known increased risk of malignant transformation of b-HCAs.
Diagnosis of HCC arising from HCA can be difficult. There are characteristic MRI findings for detection of malignant transformation, including nodule in nodule appearance on T2W and delayed washout. However, one study reported that only 48% of HCC lesions demonstrated these findings. HCC can also be diagnosed on biopsy, however, HCC can be difficult to distinguish from background HCA even on histology. Kakar et al describes atypical morphological features found in the HCA portion of tumors diagnosed as HCC arising in HCA. These features include beta catenin activation or heat shock protein 70 positivity, and gains of chromosomes 1 and 8, similar to the changes often found in HCC. This suggests that these lesions may represent very well-differentiated HCC.
One study found that in 39% of patients, despite expert review of biopsies, HCC could not be distinguished from HCA with certainty. These lesions have been described as borderline hepatocellular adenoma or atypical hepatocellular neoplasm, and some pathologists have advocated for recognition of a new classification of lesions called hepatocellular neoplasm of uncertain malignant potential (HUMP) has been suggested. The proposed definition of HUMP includes lesions with atypical pathological or clinical findings. Atypical pathological findings (Figure 1) include focal reticulin loss, focal cytological atypia in < 5% of tumor, focal architectural atypia in < 5% of tumor, and beta-catenin activated. Atypical clinical features include females older than 50 years of age, males, anabolic steroid use, or some congenital conditions (glycogen storage disease, Fanconi anemia). However, one study reported that re-classification of tumors with this criteria would result in over half of patients being diagnosed with HUMP. Notably, in the series of 21 patients, with 26 (62%) of lesions classified as HUMP, HUMP was not correlated with increased rate of HCC. Further studies are needed to clarify the classification of HUMP and its clinical significance.
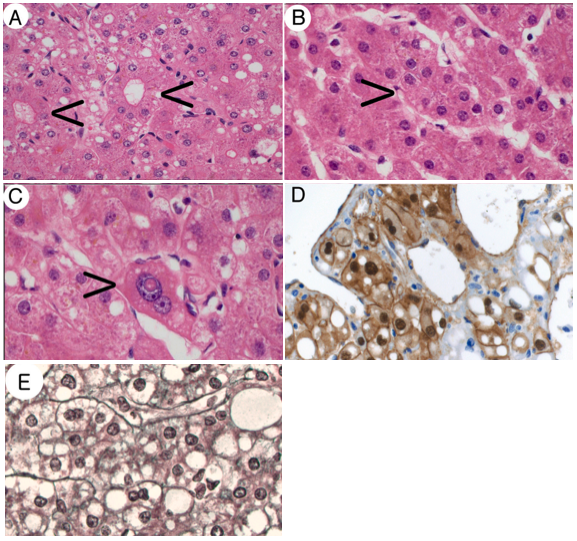
Figure 1. Adapted from Larsen et al 2021. Examples of criteria for inclusion as a well-differentiated hepatocellular neoplasm of uncertain malignant potential. A: Pseudoglandular formations (arrows) (H&E, x200). B: Focal small cell change (arrow) (H&E, x200). C: Focal cytological (nuclear) atypia (arrow) (H&E, x400). D: Nuclear β-catenin positivity (β-catenin, x400). E: Focal, mild widening of hepatocyte plates in a well-differentiated hepatocellular neoplasm with otherwise intact reticulin framework (reticulin stain, x400).
Back to the case:
While the patient’s MRI has findings characteristic of HCA, the HCA subtype is not identified. Per EASL practice guidelines, only H-HCA and I-HCA can be accurately identified on MRI. Biopsy could help to identify HCA subtype and associated malignant potential, however is not recommended unless diagnosis of HCA is unclear or if there is concern for malignant transformation. Additionally, the patient is already known to be at increased risk of HCC given male sex.
Management and Outcomes
Management of HCAs depends on presence of risk factors for complications of HCA. The American College of Gastroenterology (ACG) and European Association for the Study of the Liver (EASL) both have guidelines for management of HCAs (Table 3). Most commonly, HCAs are found in the presence of OCP use, and women are recommended to discontinue OCPs and follow up with surveillance imaging. Usually after discontinuation of OCPs, these HCAs are stable or regress in size, and no further intervention is needed. The EASL 2016 Clinical Practice Guidelines recommend all males with HCA, as well as those with biopsy proven b-HCA, to undergo intervention due to risk of malignant transformation. In females with HCA > 5 cm despite OCP discontinuation, or progressively enlarging HCA, intervention is also warranted for both risk of hemorrhage and malignant transformation. The ACG 2014 guidelines recommend intervention for HCAs > 5 cm, and do not specify different management based on sex. Treatment strategy for HCAs differs slightly based on guidelines and is determined by clinical and in some cases pathological evaluation of risk factor for complications such as hemorrhage and malignant transformation.
Surgical resection
Surgical resection is definitive therapy for HCAs. Resection is recommended for all men with HCAs due to the increased risk of malignant potential. It is also considered for HCAs that are > 5 cm or enlarging. Studies have demonstrated good outcomes after surgical resection of HCA, though the number of cases remains small. In one study of 36 patients who underwent surgical resection of 48 HCAs, 2 patients with HCA > 10 cm were diagnosed with HCC on pathology. Both had local recurrence, at 4 months and 5 years, and underwent a second intervention. None of these patients died from HCC. In a study of 118 patients who underwent surgical resection of HCA, 8% of patients had recurrence of HCA or increase in size. Of the 10 patients with malignant transformation, there was no recurrence of malignancy at mean follow up time of 78 months. Major postoperative morbidity including biliary fistula and pulmonary embolism occurred in 18 (15%) of patients. One study reported tumor recurrence rate of 40% at 5 years, though also found recurrence rate to be lower than HCC that developed in normal liver parenchyma. While surgical resection has risks, it remains the first-line therapy for patients with high-risk HCAs that require intervention.
Surgical resection has also been reported in patients with type I GSD. However, these cases are rare and severe post-operative complications have been reported. In a case series of 7 patients with type I GSD who underwent surgical resection, 2 patients died and 5 patients eventually had adenoma progression and underwent liver transplantation.
Surgical alternatives
There are a few alternatives for surgical resection, for those who are not surgical candidates or would prefer to avoid surgery. Transarterial embolization (TAE) can be effective in treating hemorrhage from HCA and in reducing tumor size, however its utility in those with malignant potential remains unclear. In one study with 25 patients (and 37 HCAs) who underwent bland TAE for HCA, the majority (23/25) had multiple HCAs or adenomatosis, and 14/25 had suspected malignancy. Of the 37 HCAs that underwent embolization, there were 3 persistent lesions that required second intervention. Five adenomas disappeared, 22 decreased in size, and 7 remained stable. In a systematic review of patients undergoing TAE for HCAs, 10% of patients had complete resolution of tumor, 75% showed tumor regression, and 14% had stable disease. No patients showed increase in tumor size. Of note, only 1 patient included in this systematic review had malignant transformation. One case report described transarterial chemoembolization in a male patient with multiple HCAs, with largest lesion measuring 7 cm, with unclear malignant potential. Follow up imaging showed decrease in size of largest lesion to 5.5 cm and stable size after 2 years. Given increased risk of malignant potential in patients with larger size HCAs, TAE may be helpful in preventing malignant transformation by decreasing tumor size, however this requires further investigation.
Radiofrequency ablation (RFA) has also been studied in management of HCA however further evaluation of efficacy is needed. RFA is an accepted treatment modality for small (<3 cm) HCCs. One study followed 18 patients who underwent RFA for HCA, all of which were women. There were a total of 76 HCAs ranging in size from 1 cm to 14 cm with median diameter of largest lesion 3.8 cm. Eight patients (44%) required one session of RFA while 56% required 2 or more sessions. At the end of the study, 7 HCAs still showed evidence of minimal residual HCA tissue adjacent to ablation zone. In one case report, 4 small (≤ 2 cm) biopsy proven inflammatory HCAs in a patient with GSD-Ia were treated with RFA follow up imaging showing no residual tumor. Ablation may be helpful in the treatment of small HCAs, however its role in treating those with risk for malignancy or suspected malignancy is unclear, especially with reported treatment failures in existing data.
Liver Transplantation
Liver transplantation (LT) has been rarely pursued for management of HCAs. EASL guidelines recommend against liver transplantation except in patients with > 10 HCAs and with underlying liver disease. The United Network for Organ Sharing (UNOS) grants exception points for HCA for (1) presence of GSD, (2) unresectable b-HCA, or (3) HCA that is unresponsive to medical management, unresectable, and progressive or with complication such as hemorrhage or malignant transformation. A retrospective analysis of the UNOS database from 1994 to 2020 included 199 patients who were listed for LT with indication of HCA. Of these patients, 118 eventually underwent LT and an additional 24 patients who underwent LT were incidentally diagnosed with HCA after LT. GSD was a co-diagnosis in 6.3% of patients. HCC was a known complication pre-LT in 7 (4.9%) patients, and incidental HCC was noted on explant in 8 (5.6%). Most patients (90.1%) received a deceased donor graft, and 61.3% of patients received exception points. The patient survival rates at 1- and 5-year follow up were 94.2% and 86.3%, respectively. Graft survival rates at 1- and 5- year follow up were 89.3% and 80.0%, respectively. Ziogas et al also conducted a systematic review of patients with HCA undergoing LT, ultimately including 99 patients, 67 of which were outside the United States. Indications for LT included suspected malignancy (39.7%), unresectable HCA (31.7%), and increasing HCA size (27%). 53.1% of patients had GSD. Malignant transformation occurred in 18 (23.1%) of patients, with 6 (7.7%) patients having pre-LT biopsy confirmed HCC, and 12 (15.4%) patients with incidental HCC on explant pathology. Most patients who underwent LT did not meet UNOS exception point criteria, and early LT evaluation for living donors may be beneficial in the future. Overall, LT for HCA remains rare and is not recommended as first-line treatment even in patients with proven malignant transformation, though post-LT outcomes are favorable.
Table 3: Guidelines for HCA management
| EASL 2016 Clinical Practice Guidelines | ACG 2014 Clinical Guideline | |
| Lifestyle changes |
|
Avoid OCP, hormone-containing intrauterine devices, anabolic steroids |
| Diagnosis |
|
|
| Indication for intervention |
|
|
| Intervention |
|
|
| Surveillance |
|
|
Back to the case:
With the increased risk of malignant transformation associated with male sex, intervention is indicated regardless of other risk factors. First-line treatment is surgical resection if he does not have contraindications. If he is determined to be a poor surgical candidate, embolization or ablation could be good options, especially given the relatively small HCA size (≤ 3 cm). Liver transplantation should only be considered if the lesion is unresectable and unresponsive to embolization/ablation.
Key Points:
- Malignant transformation of HCA is rare, with estimated rate of 4-8%.
- Risk factors for malignant transformation include male sex, size > 5 cm, β-catenin activated subtype, increased or rising AFP and type I GSD.
- It can be difficult to differentiate HCC from underlying HCA on biopsy. A new category of lesions, hepatocellular neoplasm of uncertain malignant potential (HUMP), has been suggested, though clinical significance remains unclear.
- First-line therapy for HCA with biopsy proven HCC or at high risk of malignant transformation is surgical resection.
- Liver transplantation is not routinely recommended in patients with HCA, even with biopsy proven HCC, and should only be considered if the lesion is unresectable and unresponsive to medical management.
