Malnutrition in the Adult with Cirrhosis
Malnutrition in the Adult with Cirrhosis
Malnutrition is common in patients with cirrhosis and may manifest with frailty and/or sarcopenia. Malnutrition is operationally defined as an imbalance (deficiency or excess) of nutrients that causes measurable adverse effects on tissue/body form (body shape, size, composition) or function and/or clinical outcome by the AASLD. Patients with liver disease may have undernutrition or malnutrition with obesity, both of which can cause muscle mass loss. Clinically this may present with a phenotype of frailty with impaired muscle contraction and/or sarcopenia with loss of muscle mass. Malnutrition occurs in about 20 % of patients with compensated cirrhosis and 50% of patients with decompensated cirrhosis. Resultant sarcopenia and frailty affect up to 30-70% of patients with end-stage liver disease, but these entities are often underrecognized. Malnutrition and sarcopenia are associated with higher rates of complications in cirrhosis including infections, HE, and ascites, with associated lower rates of survival.
The Physiology of Malnutrition
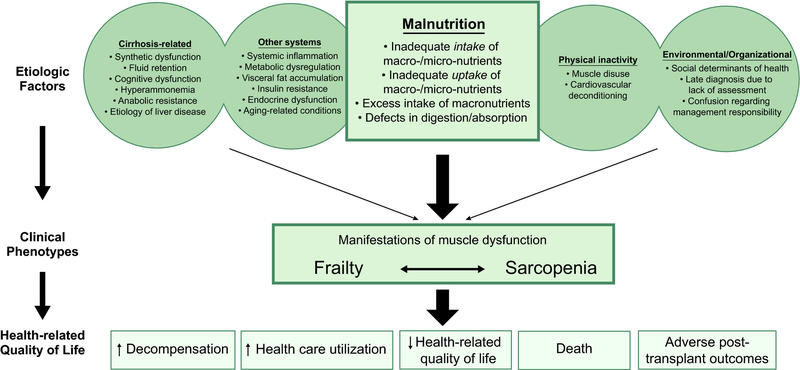
Malnutrition is the result of complex processes (Figure 1). Patients may have low intake of macronutrients related to early satiety with abdominal distention, anorexia, dysgeusia, diet unpalatability (low salt diets) and frequent fasting with repeated hospitalizations. The underlying pathology in cirrhosis itself may lead to malnutrition through chronic inflammation, altered protein metabolism, impaired myotoxic ammonia, and disease specific concerns including alcohol exposure increasing muscle autophagy, insulin resistance in NASH, and cholestasis related induction of skeletal muscle atrophy through bile acid elevation. Notably, even outside of these unique disease processes patients with cirrhosis may also have environmental or organizational risks for malnutrition, including food insecurity and food insufficiency. This multi-hit process degrades adequate intake and optimal metabolism resulting in muscle dysfunction and its consequences.
Figure 1: “Factors contributing to malnutrition, frailty, and sarcopenia and the relationship between these three constructs. Cirrhosis- related and other systems- related factors, along with physical inactivity and environmental/organizational factors, contribute to malnutrition— which then leads to frailty and sarcopenia. These factors can also contribute directly to frailty and sarcopenia independently of malnutrition” from the AASLD
Screening for Malnutrition
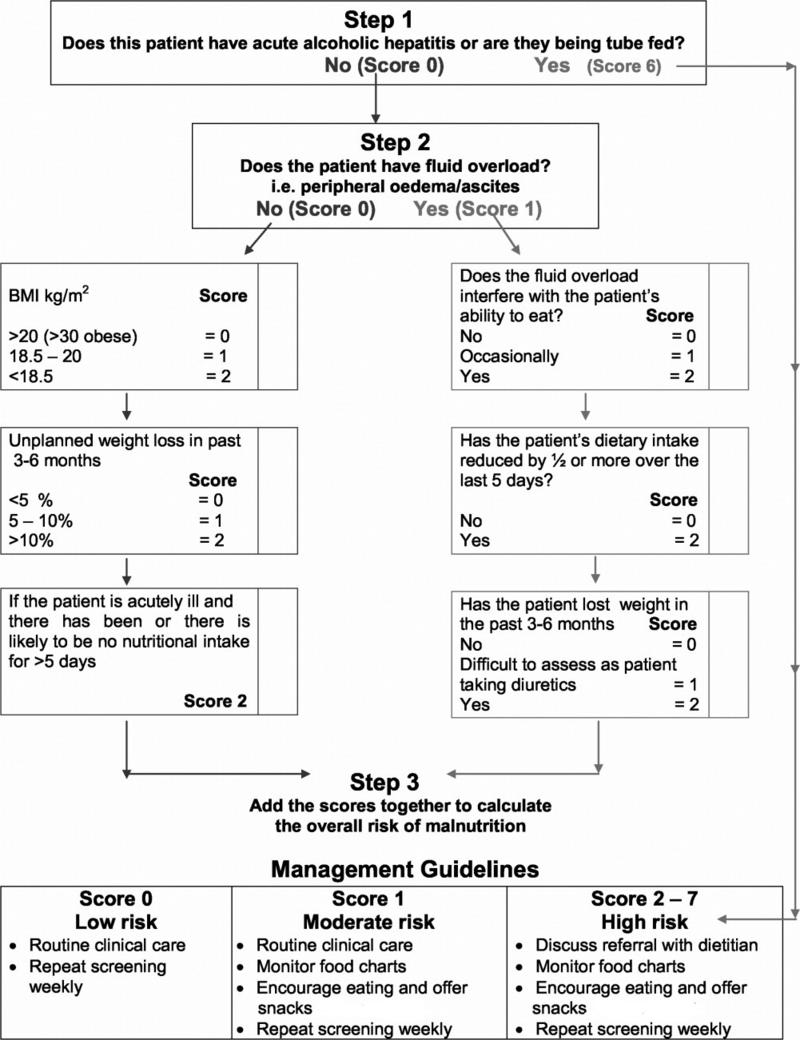
Yet, clinically recognizing malnutrition in patients with cirrhosis can be challenging. The Royal Free Hospital Nutrition Prioritizing Tool (RFH-NPT) (Figure 2), while needing further validation, can be used to screen for malnutrition among patients with cirrhosis, classifying patients into low, moderate, and high nutritional risk. It should be assumed that any patient with a BMI under 18.5 and those with advanced decompensated cirrhosis (Child-Pugh C) have high risk of malnutrition. For others, the RFH-NPT score is an independent predictor of hepatic decompensation, quality of life, and transplant-free survival. This score takes into account that patients with alcohol-associated liver disease and particularly alcohol- associated hepatitis are at a particularly high risk of malnutrition. Patients who screen positive should be referred to a dietician for a more detailed assessment including intake assessment and reassessment every few months.
Figure 2: Flow chart showing the Royal Free Hospital-Nutritional Prioritizing Tool (RFH-NPT) to determine a patient’s risk of malnutrition
Risks of Malnutrition: Frailty
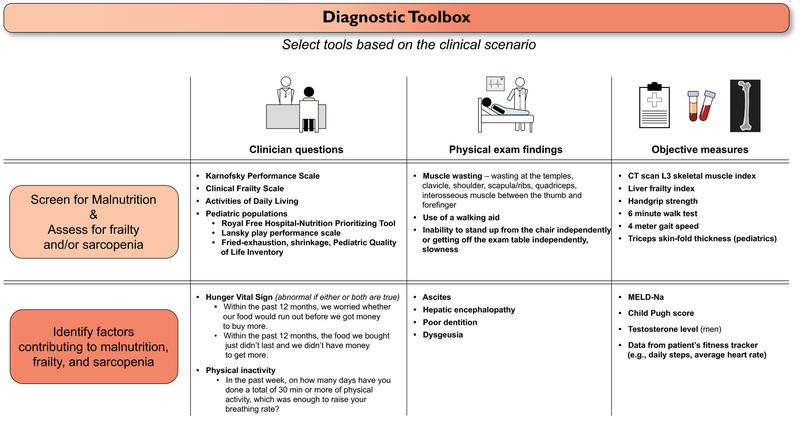
The 2021 AASLD guideline recommends patients with cirrhosis should be assessed for frailty with a standardized tool both at baseline and longitudinally. The AASLD has a helpful diagnostic toolbox below (Fig 3) which outlines questions, exam findings, and objective measures useful for diagnosing malnutrition and frailty among patients with cirrhosis.
Multiple standardized tools exist to screen for frailty and monitor severity over time. These include but are not limited to the Liver Frailty Index (LFI), The Karnofsky Performance Status (KPS), Fried Frailty Instrument, the 6-minute walk test, and more. These screening tools are often adopted from geriatric medicine.
Risks of Malnutrition: Sarcopenia
Patients with cirrhosis should also be monitored for development of sarcopenia. The gold standard to assess sarcopenia is through CT guided quantification of skeletal muscle mass. Muscles at the L3 level including the psoas muscle mass and other core muscles thought to be independent of activity level and water retention as typically used. Patients with cirrhosis often undergo CT imaging for reasons outside of sarcopenia evaluation. Whenever a CT is performed it may be possible to assess muscle mass and use this to prompt nutrition and activity assessments. The AASLD recommends reassessing frailty or sarcopenia every year in patients with compensated disease and up to every 8 weeks for those with decompensated disease.
Figure 3: Diagnostic Toolbox to screen for malnutrition, assess for frailty and/or sarcopenia and identify risk factors through clinical questions, exam findings and objective measures from the AASLD
Interventions
Patients who have screened positive for frailty or sarcopenia and those at risk for malnutrition should receive a personalized “prescription” for intake that is tailored to their individual needs. This prescription should involve management of their underlying medical conditions, boosting macronutrient intake, consideration of micronutrient intake and treatment of other systems to maximize nutrition and improve muscle mass. According to AASLD guidelines, patients should undergo multidisciplinary team management, ideally including the patient’s primary care provider, gastroenterologist/hepatologist, registered dietician, certified exercise physiologist/physical therapist, and health behavior specialist especially if they have progressive frailty or sarcopenia. Despite aggressive intervention malnutrition in cirrhosis and resultant muscle mass loss may not always be reversible.
Patients with alcohol-associated hepatitis and cirrhosis typically have increased resting energy expenditures and it may be difficult for patients to keep up with this higher caloric need. Caloric intake can be impacted by restrictive diets in cirrhosis particularly in those with ascites. Ascites itself may be associated with early satiety and decreased caloric intake. Therapeutic paracentesis has been shown to improve anorexia for some patients. Patients on a low salt diet may struggle to meet intake needs due to diet unpalatability. Each patient’s current risk of malnutrition should be balanced with the moderate advantage of salt restriction in the treatment of ascites and liberalizing salt restriction should be considered to boost caloric intake.
Prior historical concerns that hepatic encephalopathy could worsen with protein intake have not been replicated in recent studies. A randomized control trial by Córdoba et al. changed this dogma in 2004 with 30 hospitalized patients on protein restriction demonstrating higher protein catabolism compared to those on normal protein diets without a difference in evolution of HE between the groups. Protein sources remain controversial with consensus being that incorporating vegetable and dairy-based protein sources instead of solely meat-based protein is beneficial in HE.
Notably, patients with cirrhosis may also be impacted by “NPO” time prior to procedures like outpatient paracenteses or during hospitalizations. It is important to recognize patients with cirrhosis continue to have high energy expenditures in the hospital and time spent without intake should be minimized to avoid periods of fasting over 3-4 hours as able.
Patients with cirrhosis are prone to vitamin and mineral deficiencies. Patients with alcohol-associated liver disease may have vitamin D, E, folate, thiamine, zinc, and selenium deficiencies. Patients with cholestatic liver disease are at risk for fat-soluble vitamins A, E, D, and K deficiencies. DEXA scans should be performed for those with cholestatic liver disease, on chronic steroids, or alcohol use disorder and all patients with a T-score below -1.5 should start calcium and vitamin D supplementation. Vitamin D and zinc deficiencies may be associated with sarcopenia. Dysgeusia may be associated with zinc deficiency further worsening malnutrition due to taste abnormalities. Magnesium is often low related to diuretic use and malabsorption. Repletion of vitamins and minerals should be considered for those with a deficiency and monitoring after repletion for ongoing needs annually may be useful.
Interventions to improve nutrition, frailty and sarcopenia may consist of a combination of the following:
Management of Cirrhosis and Underlying Conditions
- Disease specific – treatment of inflammatory conditions causing cirrhosis including curing HCV and alcohol cessation through behavioral and pharmacotherapies
- Complication specific – treat complications of cirrhosis as you otherwise would to improve overall health. Specific complications may have impacts on nutrition, frailty, and sarcopenia:
- Ascites- Medical therapies to limit early satiety associated with abdominal distension and improve mobility. Therapeutic paracentesis may be needed.
- HE- Consider lactulose and rifaximin. Theoretical basis that decreasing ammonia may be beneficial for management of sarcopenia and frailty. Note limited data in humans.
- Portal hypertension – TIPS if necessary for other indications may improve muscle mass. Liver transplantation may improve frailty and/or sarcopenia in some patients. Neither can be recommended solely for improvement of frailty/sarcopenia.
Intake Interventions – Macronutrients
- Caloric intake for patients with normal BMI should target at least 35 kcal/body weight/day
- Caloric intake for stable non-hospitalized patients with elevated BMI should be individualized with general target 25-35 kcal/kg/body weight/day for BMI 30-40 kg/m2 and 20-25 kcal/kg/day for persons with BMI over 40. Consider the confounding effect of fluid overload in those with elevated BMI.
- Liberalization of sodium restriction should be considered if a patient is unable to maintain nutritional targets
- Adults with cirrhosis who are clinically stable should intake 1.2-1.5 g protein/kg IBW/day. Those with sarcopenia should target 1.5 g/kg IBW/day.
- Adults with cirrhosis who are critically ill should target 1.2-2.0 g protein/kg IBW/day
- Protein intake should NOT be restricted in patients with HE though protein should come from a range of protein sources (vegetable, dairy encouraged)
- Fasting time should be minimized with maximum 3-4 hours between nutritional intake during waking hours, with a goal of 3-5 meals per day
- An early breakfast and/or late-evening snack should be recommended
- Esophageal varices are not an absolute contraindication for nasogastric tube placement
- Parenteral nutrition may be needed for those patients who cannot be nourished through oral or enteral routes
Intake Interventions – Micronutrients
- Assess micronutrient deficiencies annually and replete if deficient
- Supplement vitamin D orally if <20ng/ml to reach >30 ng/ml
Other Interventions
- Physical activity can improve contractile function and muscle mass in patients with cirrhosis
- A combination of aerobic and resistance exercises should be recommended but tailored to a patient’s baseline and modified in intensity as necessary
- Consideration of referral for dental work as needed
- Testosterone replacement in male patients with low testosterone should be carefully considered (likely avoid in those with HCC, malignancy, prior thrombosis, women)
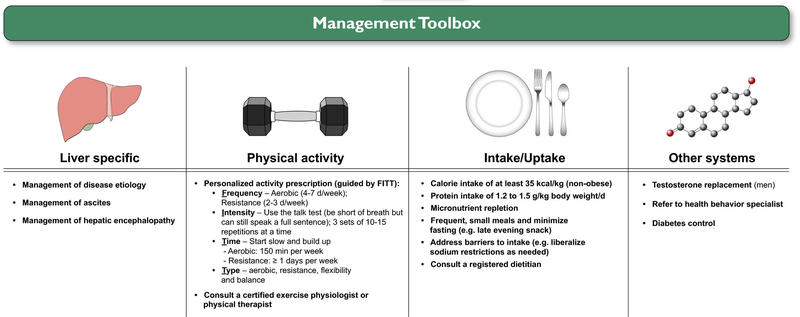
See the helpful Management Toolbox below from the AASLD for guidance:
Figure 4: Management Toolbox regarding specific interventions malnutrition, frailty and/or sarcopenia though targeting liver specific interventions, physical activity, intake and uptake-based interventions, and those interventions aimed at other systems from the AASLD
Patient education
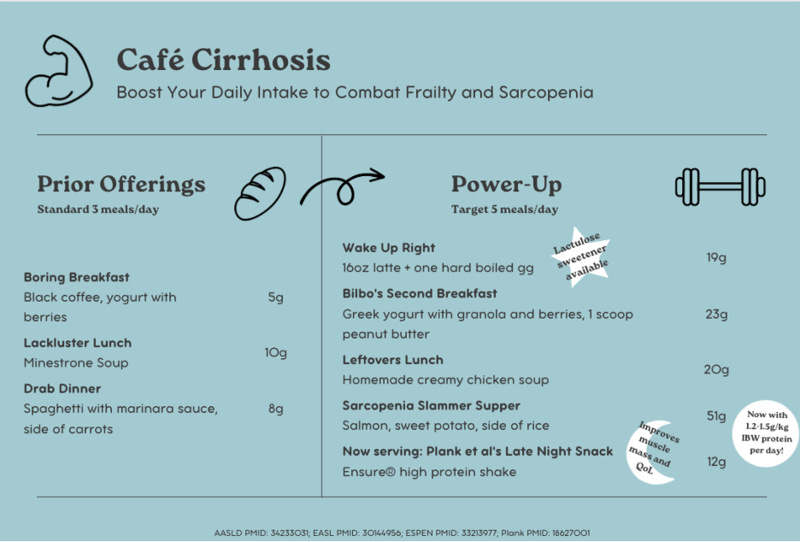
Many patient-focused resources exist to help explain the key role nutrition plays in cirrhosis. Some initial resources are below which can be helpful to start the brainstorming process for helpful meal adjustments, ways to avoid fasting, and ways to increase protein intake. Check out the Café Cirrhosis menu for some initial ideas!
EASL also has a helpful short outpatient dietary advice tool for use in the outpatient setting:
Other helpful resources:
CirrhosisCare – Liver Friendly Cooking https://cirrhosiscare.ca/healthy-living-patients/liver-friendly-cooking/
CirrhosisCare.ca- Cirrhosis Nutrition Info https://cirrhosiscare.ca/healthy-living-patients/cirrhosis-nutrition-info/