Back to Basics: Iron Overload Unmasked
Learning Objectives:
In this Back to Basic post, the learner will be able to:
1. Review the physiology of iron absorption.
2. Differentiate between primary and secondary iron overload.
3. Identify clinical features of iron overload.
4. Diagnostic approach to suspected iron overload.
1. Iron absorption refresher:
The physiology consists of four steps: I-R-O-N:
1. Intake
2. Redistrution
3. Onward Transport
4. Normalization
First Step: Intake
Dietary iron exists in two forms: I) heme iron and II) non-heme iron.
I. Heme iron is absorbed intact by enterocytes via a heme transporter.
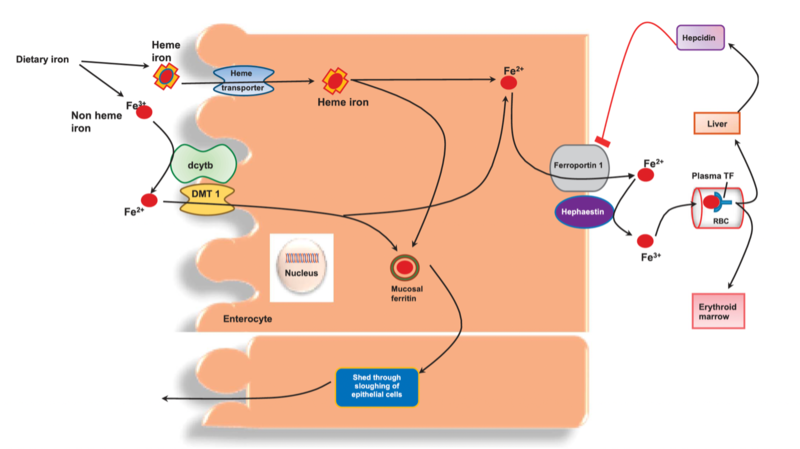
II. Non-heme iron is predominantly absorbed in its ferrous (Fe2+) form. It undergoes reduction facilitated by duodenal cytochrome b (DCYTB) and is subsequently transported into enterocytes by the divalent metal transporter 1 (DMT1). Figure 1 summarizes this.
Second Step: Redistribution
Once inside enterocytes, iron can follow two pathways:
• Storage: Iron can be stored as ferritin, a cytoplasmic protein that sequesters excess iron, preventing cellular damage from oxidative stress.
• Export: Iron can be transported across the basolateral membrane of enterocytes into the bloodstream by ferroportin, the sole known iron exporter.
Third step: Onward Transport
• In the bloodstream, ferric iron binds to transferrin (TF), a glycoprotein iron carrier. Iron-loaded transferrin delivers iron to various tissues and organs, including the bone marrow for erythropoiesis, the liver for storage, and other cells for metabolic processes.
Fourth Step: Normalization
• Hepcidin, a peptide hormone synthesized in the liver, plays a central role in iron homeostasis by regulating ferroportin expression. Increased hepcidin levels lead to ferroportin degradation, reducing iron absorption and recycling, whereas decreased hepcidin levels enhance iron release into circulation represented in Figure 1.
Figure 1: Iron hemeostasis. Dcytb is duodenal cytochrome b. DMT1 is divalent metal transporter 1. TF is transferrin. Fe2+ is ferrous and Fe3+ is ferric iron.
2. Classification of iron overload
The 2011 AASLD guidelines classify patients with iron overload syndromes into three groups:
1. Primary – patients with genetic disorders that can cause iron overload.
2. Secondary – patients with iron overload as a result of excess absorption and organ deposition, but unrelated to inherited causes of iron overload.
3. Miscellaneous – patients with rare diseases such has neonatal iron overload, aceruloplasminemia, and congenital atransferrinemia.
Primary iron overload
• Primary iron overload disorders are defined as inherited conditions, with hereditary hemochromatosis (HH) as the most identified genetic disorder. Prevalence in the US is 1:200 to 1:500 for homozygous mutations, seen mostly in Caucasians (highest in Scandinavian or Irish descent, lowest in African descent).
• The primary defect is missense mutation in the HFE gene resulting in a cysteine to tyrosine substitution at aminoacidic 282 (C282Y), a protein that interacts with transferrin receptor 1 (TfR1) and regulates the expression of hepcidin, a key regulator of iron metabolism represented in Diagram B of Figure 2.
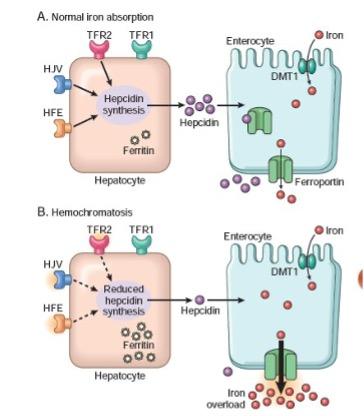
Figure 2: Normal iron absorption (A) versus hemochromatosis (B).
• C282Y homozygotes account for 80-95% of patients with HH also know as type 1A. Another genetic subtype is the H63D or type 1B mutation that may act as a cofactor for phenotypic expression of iron overload, primarily in combination with C282Y. A third HFE genotype is related to the mutation S65C that may lead to increased serum iron and ferritin levels. Table 1 summarizes subtypes of hemochromatosis, genes involved, and clinical manifestations
• Overall reduced hepcidin levels result in the upregulation of ferroportin expression on enterocytes, hepatocytes, and macrophages, leading to increased iron efflux into circulation. Consequently, more iron is absorbed from the diet and released from iron stores, contributing to progressive iron accumulation in tissues.
• The risk of developing cirrhosis rises significantly with elevated serum ferritin (SF) levels, usually >1,000 ng/mL at diagnosis.
• It is possible for patients who are heterozygous for HFE mutations namely C282Y to develop hepatic iron deposition, and accelerate the development of clinical liver disease in the setting of multifactorial hepatic risk factors (steatotic liver disease, viral hepatitis, etc.).
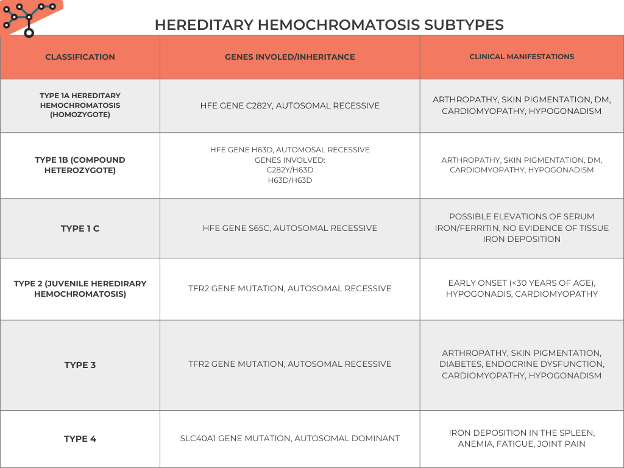
Table 1: Back to Basics: Hereditary Hemochromatosis subtypes.
Secondary iron overload
Secondary iron overload is a phenomenon of excessive absorption and subsequent organ deposition, which is unrelated to a known genetic mutation:
• Chronic liver Diseases such as hepatitis C and B, alcohol liver disease, MASLD, and porphyria cutanea tarda downregulate hepcidin, resulting in increased iron absorption from the gastrointestinal tract.
• Alcohol use disorder: Alcohol suppresses hepcidin synthesis in parenchymal cells of the liver. Due to its crucial role in the regulation of body iron stores, hepcidin may act as a secondary risk factor in the progression of alcohol-associated liver disease.
• Hemolysis: Disorders such as hereditary spherocytosis, sickle cell disease, or autoimmune hemolytic anemia can lead to chronic hemolysis and subsequent iron overload. Free hemoglobin released from lysed red blood cells is scavenged by haptoglobin and processed by macrophages, leading to iron accumulation in reticuloendothelial cells and, in severe cases, systemic iron overload.
• Inflammatory states includes systemic lupus erythematosis, rheumatoid arthritis, and malignancies like hepatocellular carcinoma. In these states, pro-inflammatory cytokines such as interleukin-6 (IL-6) stimulate hepcidin production in the liver. Ferritin can be an acute phase reactant. Elevated levels of hepcidin lead to downregulation of ferroportin, the iron exporter protein, reducing iron absorption and recycling.
3. Clinical features and liver damage
• Most patients with hereditary hemochromatosis are asymptomatic for many years, and clinical manifestations usually do not appear until the fifth or sixth decade of life. 18% of men and 5% of women are asymptomatic.
• The clinical disease tends to occur more commonly in men than pre-menopausal women, who are protected from iron accumulation during menstruation and pregnancy.
• Clinical manifestations are varied, reflecting both the direct effects and secondary complications of iron overload. Common features include weakness, abdominal pain, and loss of libido as the three most common patient-reported symptoms (Figure 3).
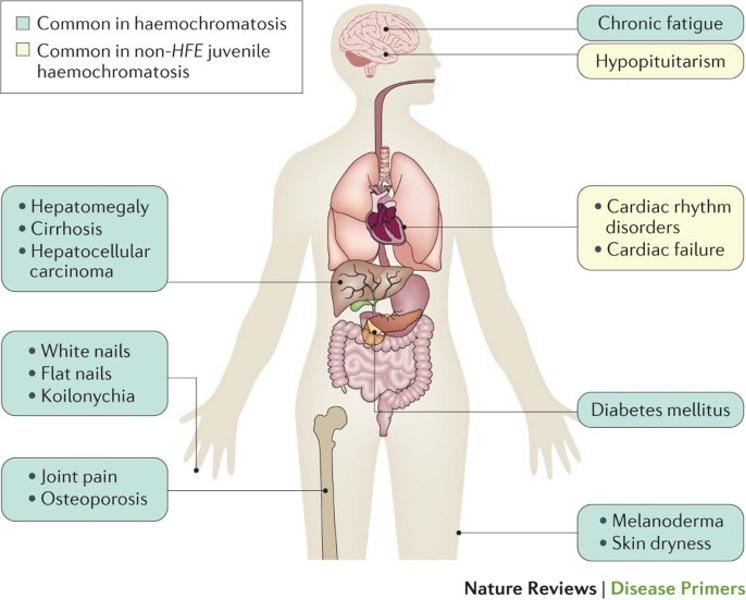
Figure 3: Principal clinical features in hereditary hemochromatosis.
• The liver is the most affected organ in patients with hemochromatosis and secondary iron overload. Clinical presentation can include asymptomatic elevation of serum aminotransferases, nonspecific right upper quadrant pain, or complications of end-stage liver disease.
• Rates of cirrhosis increase by 9-fold if there is concurrent alcohol use. Decreasing alcohol intake can decrease phenotypic expression of disease.
4. Approach to suspected iron overload
Based on 3 steps (Figure 4):
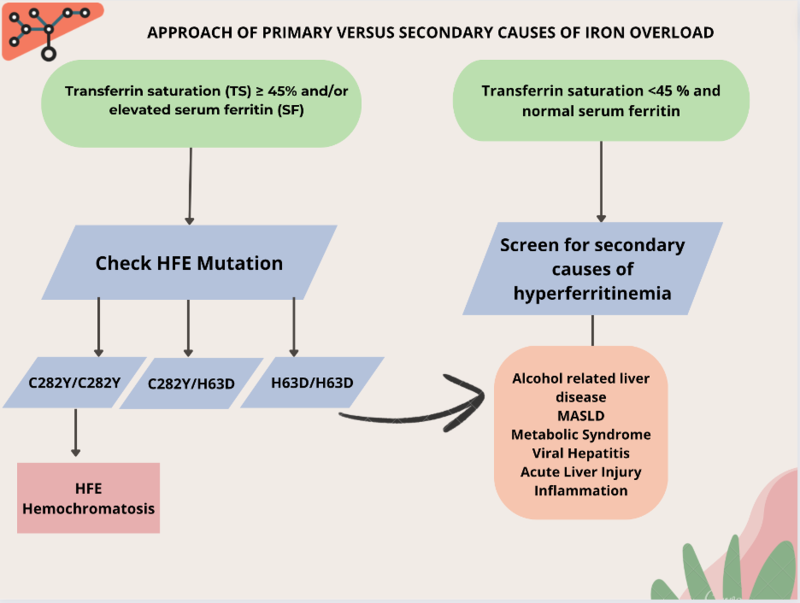
Step 1: Obtain a transferrin saturation (TS) and serum ferritin (SF).
Step 2: Is the TS ≥ 45% and/or SF elevated? Yes or No?
Step 3: If TS ≥ 45%, perform HFE gene testing; if TS < 45%, exclude secondary causes.
Figure 4: Approach of primary versus secondary iron overload.
• Transferrin saturation (TS) is the preferred initial screening test. A TS of greater than 45% identifies 97.9%-100% of C282Y homozygotes, although a small proportion of patients with HH may have TS of <45%.
• Liver biopsy is recommended to stage the degree of liver disease in C282Y homozygotes or compound heterozygotes if liver enzymes are elevated or if ferritin is >1,000 ng/mL. Patients should be referred to Hepatology for further evaluation and treatment.
• MRI with T2-weighted imaging can be used to estimate hepatic iron concentration non-invasively, but requires special additional software. Transient elastography (Fibroscan) is not validated to assess fibrosis stage in hemochromatosis.
Take-home points:
✔️ The physiology of iron absorption consists in 4 steps: I-R-O-N. (Intake, Redistribution, Onward transport, Normalization).
✔️ The laboratory findings of hemochromatosis usually occurs before clinical manifestations.
✔️ Not all elevated iron is due to hereditary hemochromatosis. Pay special attention to secondary causes of iron overload, such as inflammatory states and hematologic disorders.
✔️ Other forms of primary liver disease can potentiate cirrhosis in patients with hemochromatosis with homozygous or heterozygous mutations.