Nutritional Management of the Child with Cirrhosis
Overview of Nutritional Management of Child with Cirrhosis
The main factors contributing to malnutrition in cirrhotic patients are detailed in Figure 1. These are similar to adult population but are accentuated in children who have higher metabolic demand for growth.
Figure 1: Pathophysiology of malnutrition in cirrhosis
Management should include:
1. Thorough Nutrition Assessment
- Anthropometric data- length and weight every 1-4 weeks (may be falsely elevated due to ascites), Mid-upper arm circumference (MUAC) and triceps skin fold every 4-6 weeks.
- Dietary assessment- formula intake, MCT intake (if taking as supplement or high percentage formula), vitamin/trace elements/mineral intake.
- Energy expenditure- level of activity of 6 month old with encephalopathy is different than an active toddler with varices secondary to portal hypertension.
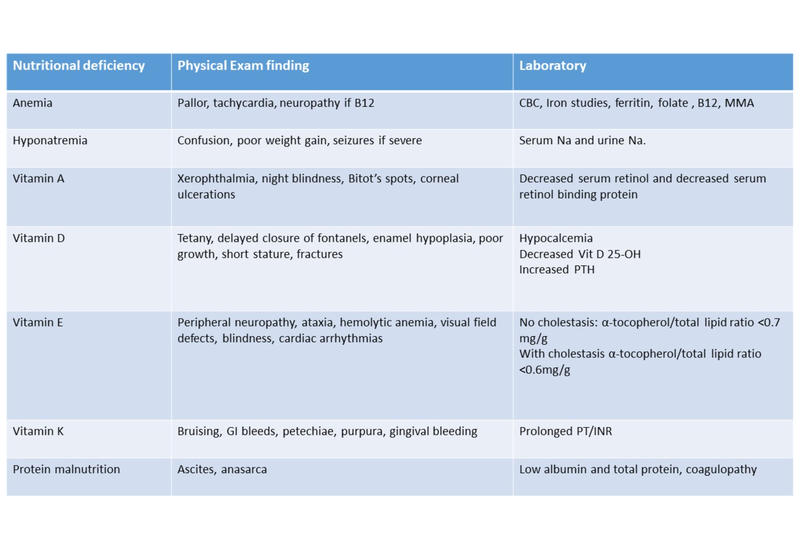
- Clinical and laboratory assessment of fat-soluble vitamin deficiencies, anemia, electrolytes and protein malnutrition. (see Table 1)
Table 1: Laboratory Evaluation for Nutritional Deficiencies
2. Determine what the daily needs are and adjust recommendations based on growth/weight gain, anthropometrics and laboratory response. In general, pediatric cirrhotic patients have the following requirements:
- Energy
- <12 months: 150% estimated energy requirement (EER)
- Toddlers/school age/teens: 120-170% EER
- Total Fat: 30-60% of total daily energy intake
- MCT 30-70% of total fat for cholestatic ESLD
- Polyunsaturated fatty acids (PUFA): >10% of total daily energy intake
- Essential fatty acids (EFA): 4.5-10.8% of total daily energy should be in form of EFA
- Carbohydrates: 40-60% of total daily energy intake
- Important to keep carbohydrate intake in short intervals to prevent hypoglycemia
- Protein: 3-4g/kg/day
- Specific supplementation with branched chain amino acids (BCAA) can be considered
- Fat soluble vitamins:
- Vitamin A: 1000 IU/kg/d (max 25,000 IU)
- Monitor levels q4w until levels are replete
- Vitamin D: 400 IU/d or higher depending on level of deficiency
- <10 ng/mL: 5000 IU/d
- 11-19 ng/mL: 4000 IU/d
- 20-29 ng/mL: 3000 IU/d
- Vitamin E: TPGS (d-α tocopherol polyethylene glycol succinate) preparation 15-25 IU/kg/d
- Vitamin K: 2.5-5 mg/d 2-7 times a week orally. May need to be given IV/SQ if poor absorption is present secondary to cholestasis. If having persistent coagulopathy, Check INR every 1-7 days depending on clinical judgment.
- Calcium: 25-100mg/kg elemental calcium per day in divided doses
- Zinc: 1mg/kg/d elemental zinc as zinc sulfate
- Selenium: 1–2 µg/kg/d
- Iron: 5-6mg/kg/d elemental iron in 3 divided doses
- Vitamin A: 1000 IU/kg/d (max 25,000 IU)
3. Create a nutrition plan in conjunction with dietician
- When developing the feeding plan, you should attempt to develop a plan that compensates for anorexia, increased energy needs, malabsorption, and abnormal use of energy sources as detailed in Figure 1.
- Children with weight-for-length z scores <–3 meet the criteria for severe malnutrition and increased risk for death.
- While attempts can be made to initially concentrate formula and increase PO caloric intake, clinicians should have a low threshold to start tube feedings.
- NG tube feeding may be safely used without increased risk for esophageal variceal bleeding in patients with pHTN.
- If tube feedings do not improve nutritional status, parenteral nutrition (PN) can be considered. This article shows PN can be provided in a relatively safe manner and improve anthropometric measurements prior to transplantation.
- Children with cirrhosis often require frequent feedings to prevent hypoglycemia.
- Gastrostomy tube placement is not recommended because of risk of GI bleed due to pHTN.
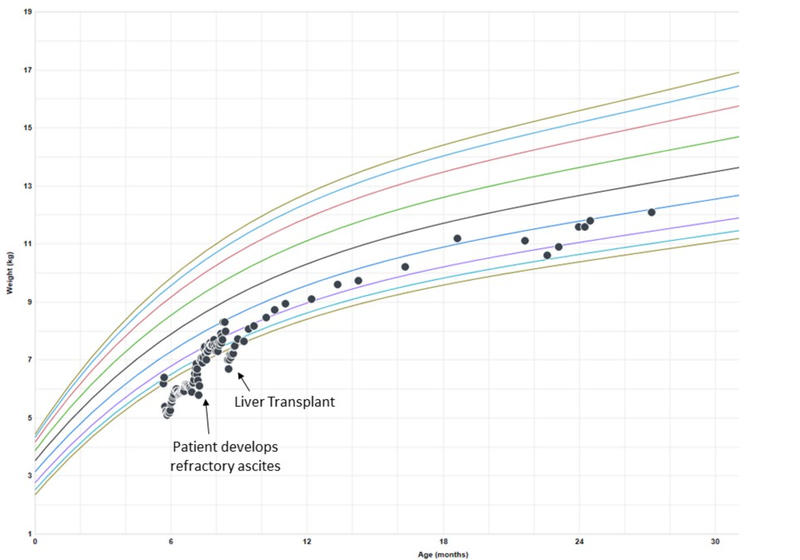
Figure 2: Example of weight growth chart
References:
- Anastacio, L. R., and M. I. Davisson Correia. "Nutrition Therapy: Integral Part of Liver Transplant Care." World J Gastroenterol 22, no. 4 (Jan 28 2016): 1513-22.
- Carpenter, A., V. L. Ng, K. Chapman, S. C. Ling, and M. Mouzaki. "Predictive Equations Are Inaccurate in the Estimation of the Resting Energy Expenditure of Children with End-Stage Liver Disease." JPEN J Parenter Enteral Nutr 41, no. 3 (Mar 2017): 507-11.
- Cheung, K., S. S. Lee, and M. Raman. "Prevalence and Mechanisms of Malnutrition in Patients with Advanced Liver Disease, and Nutrition Management Strategies." Clin Gastroenterol Hepatol 10, no. 2 (Feb 2012): 117-25.
- Juakiem, W., D. M. Torres, and S. A. Harrison. "Nutrition in Cirrhosis and Chronic Liver Disease." Clin Liver Dis 18, no. 1 (Feb 2014): 179-90.
- Mazurak, V. C., P. Tandon, and A. J. Montano-Loza. "Nutrition and the Transplant Candidate." Liver Transpl 23, no. 11 (Nov 2017): 1451-64.
- Mouzaki, M., V. Ng, B. M. Kamath, N. Selzner, P. Pencharz, and S. C. Ling. "Enteral Energy and Macronutrients in End-Stage Liver Disease." JPEN J Parenter Enteral Nutr 38, no. 6 (Aug 2014): 673-81.
- Ooi, P. H., S. M. Gilmour, J. Yap, and D. R. Mager. "Effects of Branched Chain Amino Acid Supplementation on Patient Care Outcomes in Adults and Children with Liver Cirrhosis: A Systematic Review." Clinical Nutrition ESPEN 28 (2018): 41-51.
- Smart, K. M., G. Alex, and W. Hardikar. "Feeding the Child with Liver Disease: A Review and Practical Clinical Guide." J Gastroenterol Hepatol 26, no. 5 (May 2011): 810-5.
- Sundaram, S. S., C. L. Mack, A. G. Feldman, and R. J. Sokol. "Biliary Atresia: Indications and Timing of Liver Transplantation and Optimization of Pretransplant Care." Liver Transpl 23, no. 1 (Jan 2017): 96-109.
- Utterson, E. C., R. W. Shepherd, R. J. Sokol, J. Bucuvalas, J. C. Magee, S. V. McDiarmid, R. Anand, and Group Split Research. "Biliary Atresia: Clinical Profiles, Risk Factors, and Outcomes of 755 Patients Listed for Liver Transplantation." J Pediatr 147, no. 2 (Aug 2005): 180-5.
- Wendel, D., M. Mortensen, A. Harmeson, M. L. Shaffer, E. Hsu, and S. Horslen. "Resolving Malnutrition with Parenteral Nutrition before Liver Transplant in Biliary Atresia." J Pediatr Gastroenterol Nutr 66, no. 2 (Feb 2018): 212-17.
- Yang, C. H., B. J. Perumpail, E. R. Yoo, A. Ahmed, and J. A. Kerner, Jr. "Nutritional Needs and Support for Children with Chronic Liver Disease." Nutrients 9, no. 10 (Oct 16 2017).