Pathology Pearls: Amyloidosis
Brief Case Presentation
A 70-year-old patient with a history of IgG lambda light chain amyloidosis was referred to Hepatology for persistently elevated alkaline phosphatase and ascites. Liver elastography was performed showing increased stiffness concerning for possible cirrhosis.
Select laboratory values are as follows:
| Lab Test | Result |
| AST | 24 U/L (0 – 39 U/L) |
| ALT | 19 U/L (0 – 52 U/L) |
| Alk Phos | 185 U/L (49-139 U/L |
| Total Bilirubin | 0.5 mg/dL (0.2 – 1.2 mg/dL) |
| Albumin | 4.2 g/dL (3.5-5.7 g/dL) |
A liver biopsy was performed to further evaluate for possible etiology of chronic liver disease and degree of fibrosis.
Liver Core Biopsy Findings
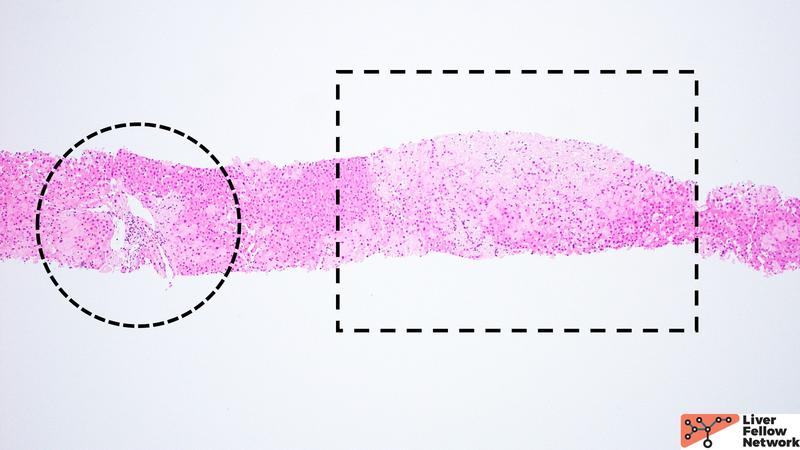
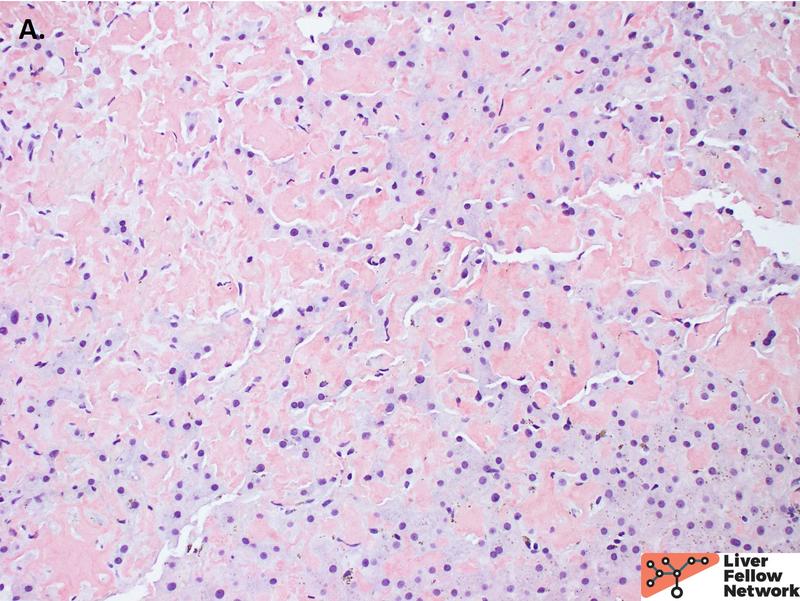
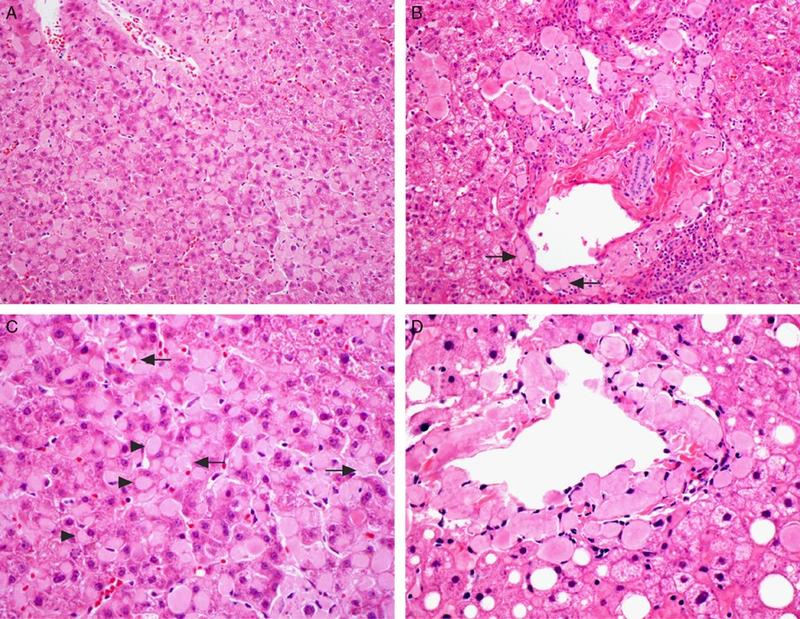
At low power, the biopsy core shows liver parenchyma with diffuse involvement by amorphous pale pink material both within the sinusoidal spaces and involving the portal tracts (Figure 1).
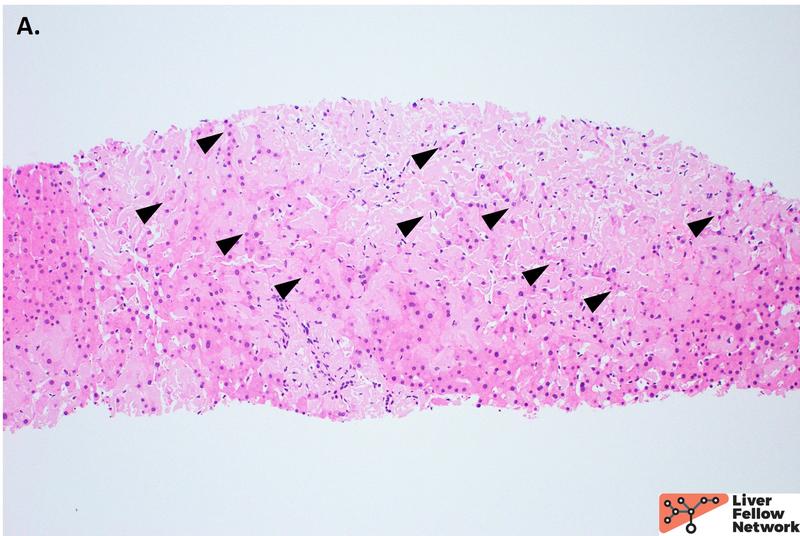
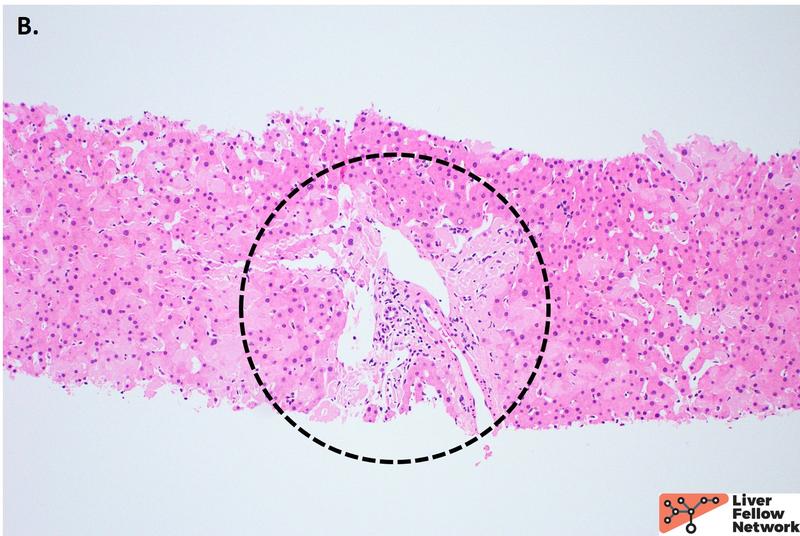
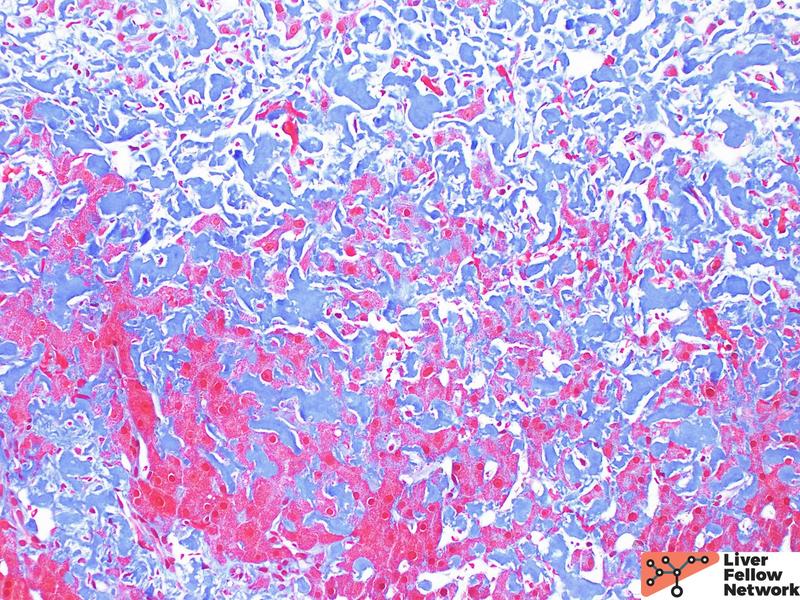
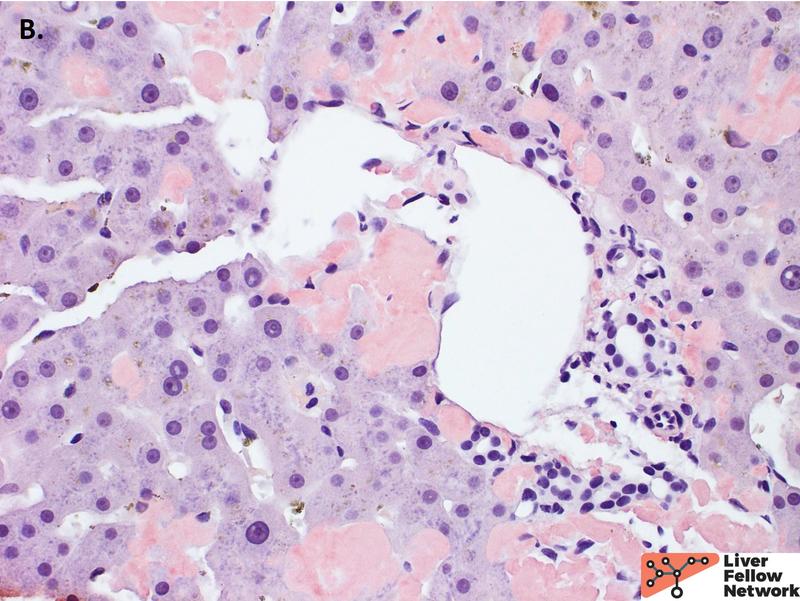
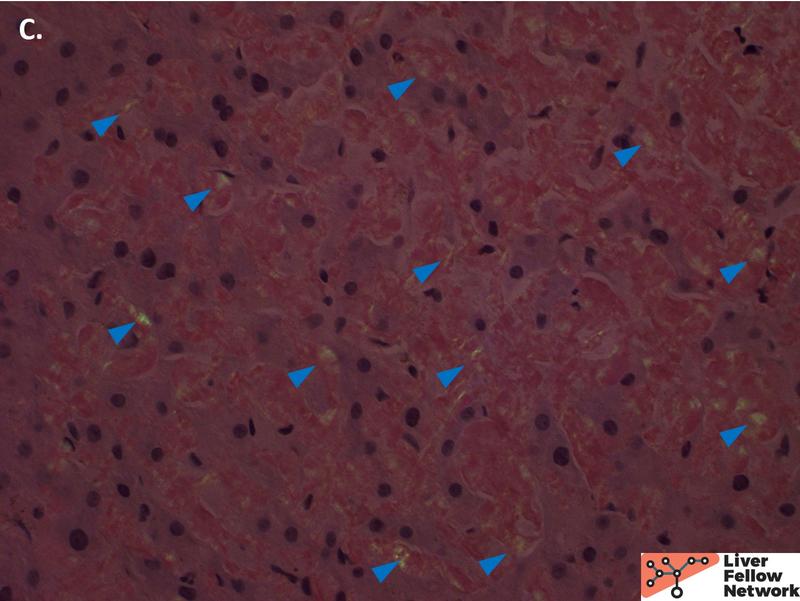
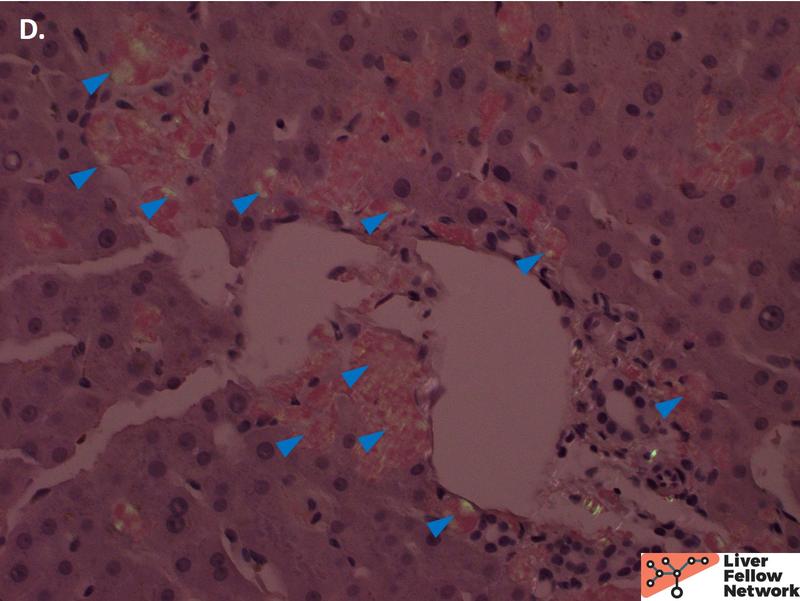
On higher power, the amorphous material distends the sinusoids causing atrophy of the hepatocellular plates (Figure 2A), with patchy involvement of the portal tracts (Figure 2B). Masson trichrome was evaluated for evidence of fibrosis. The amorphous material stains a paler blue on Masson trichrome compared to true collagen deposition/fibrosis (Figure 3). Congo Red stain was performed to further evaluate the deposits, which shows red-orange positive staining under non-polarized light (Figure 4A and 4B). With polarization, the deposits show “apple green” birefringence, confirming the presence of amyloid within the liver (Figure 4C and 4D).
Overall, the histologic findings are compatible with liver involvement by systemic amyloidosis.
Amyloidosis
Amyloidosis is a heterogenous systemic disorder characterized by deposition of insoluble fibrils within tissues as a result of abnormal protein folding. These fibrils interfere with tissue function and thus overall organ function. Type of fibrils deposited determines the systemic amyloidosis type (Table 1). Amyloid deposition in the liver is common in patients with systemic amyloidosis. Isolated hepatic involvement is otherwise rare.
Amyloidosis TypeFibril Precursor ProteinAssociation
ALImmunoglobulin light chainsPlasma cell or B-cell neoplasms
AASerum amyloid A (SAA)Prolonged chronic inflammatory conditions
ATTRWild-type or mutant transthyretinSystemic senile amyloidosis (wild-type form) and hereditary amyloidosis (mutant form)
ALECT2Leukocyte cell-derived chemotaxin 2 Pathogenesis unknown and predominantly causes renal manifestations with common subclinical liver involvement
Aβ2 M β2 microglobulin Long-term dialysis
Clinical presentation is variable and depends on site(s) of involvement. Common symptoms are nonspecific and include weight loss, fatigue, abdominal pain, anorexia, early satiety, and/or nausea. On physical exam, patients may exhibit hepatosplenomegaly, ascites, and/or edema. Extrahepatic symptoms are often present. Laboratory tests typically show elevated alkaline phosphatase and approximately one third of cases with mild elevation of liver transaminases.
In the majority of cases, amyloidosis is typically diagnosed from biopsy of fat, intestine, kidney, or bone marrow. Amyloidosis can be associated with potentially life-threatening hemorrhage due to increased vascular fragility, impaired vasoconstriction, and acquired Factor X deficiency. Due to this increased risk of bleeding or liver rupture, liver biopsy is usually not performed in patients with known amyloidosis.
Histologic Features of Amyloidosis
As with other sites within the body, amyloid deposition in the liver presents as acellular eosinophilic amorphous material. It can be found within the sinusoids and/or vessel walls. Sinusoidal deposition can compress the hepatocytes leading to hepatocellular atrophy and can efface the overall architecture. Macrophages and multinucleated giant cells may also be seen surrounding amyloid deposits. Congo red special stain performed on 10 μm thick sections is gold standard for diagnosis. Amyloid will show red-orange positive staining under non-polarized light. Under polarized light, best viewed in a darkened room, a positive Congo red stain will demonstrate the characteristic “apple green” birefringence. Falsely negative Congo red can be due to technical issues such as performing the stain on the incorrect thickness of the section. Falsely negative birefringence can be due to user-related issues such as insufficient brightness of the microscope light source or too much ambient light.
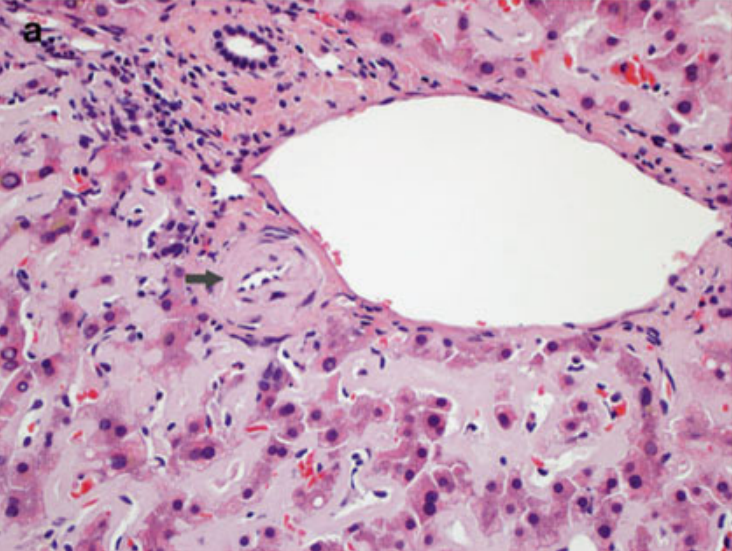
While sinusoidal deposition is more associated with AL amyloidosis and vascular pattern with AA amyloidosis (Figure 5), there can be overlap between the distribution patterns and should not be relied on to distinguish between AL and AA amyloidosis. Globular deposits within the sinusoids, however, are associated with LECT2 amyloidosis (Figure 6). Immunohistochemical stains can be used to assist in amyloid subtyping, but are often difficult to interpret due to high background staining. For definitive amyloid subtyping, laser microdissection and mass spectrometry can be performed on paraffin-embedded tissue or cytology material with up to 98% sensitivity and 100% specificity. The affected areas are removed via laser microdissection. Protein digestion is subsequently performed, followed by liquid chromatography tandem mass spectrometry (LC-MS/MS). Mass spectra detected are then compared to a protein sequence database for protein identification and subtyping.
Differential Diagnosis
While the differential diagnosis for amyloid deposition is limited, there are a few other considerations. Congo red negative, amyloid-like material within the liver can be seen in Waldenström macroglobulinemia (deposition of IgM heavy chains) or light chain deposition disease (deposition of light chains). Other extracellular material can also look similar as amyloid on H&E such as collagen, blood/fibrin, and elastosis; all of which are negative for Congo red and do not exhibit “apple green” birefringence under polarized light. Furthermore, collagen will stain strongly positive (blue) on Masson trichrome whereas amyloid is either negative or weakly positive (pale grey blue).
References
- Burt, MacSween’s Pathology of the Liver: Seventh Edition. 2017.
- Torbenson, Surgical Pathology of the Liver. 2018.
- Torbenson, Atlas of Liver Pathology: A Pattern-Based Approach. 2019
- Cummings OW, Benson MD. Amyloidosis of the gastrointestinal tract and liver. In: Picken MM, Herrera GA, Dogan A, eds. Amyloid and Related Disorders. Springer International Publishing; 2015:413-435.
- Chandan VS, Shah SS, Lam-Himlin DM, et al. Globular hepatic amyloid is highly sensitive and specific for lect2 amyloidosis. American Journal of Surgical Pathology. 2015;39(4):558-564.