Pathology Pearls: Granulomatous Liver Diseases
Brief Case Presentation
A 45-year-old female is undergoing evaluation for incidentally discovered hepatomegaly and liver function tests abnormalities. Her physical exam reveals multiple small papules around her nose and eyelids but is otherwise unremarkable. Results from her liver function tests and serologic studies are seen below:
| Lab Test | Result |
| AST | 35 U/L (8 – 37 U/L) |
| ALT | 30 U/L (8 – 35 U/L) |
| Alk. Phos. | 410 U/L (50 – 150) U/L |
| Total Bilirubin | 0.7 mg/dL (0.1 – 1.0 mg/dL) |
| GGT | 205 U/L (5 – 40 U/L) |
| HAV/HBV/HCV | |
| QuantiFERON-TB Gold Plus | NegNegativeative |
| Histoplasma Antigen | Negative |
| ANA | Positive |
| Mitochondrial M2 Ab | Negative |
| Smooth Muscle Ab | Negative |
| IgG | 1500 mg/dL (800 – 1700 mg/dL) |
| IgM | 100 mg/dL (50 – 320 mg/dL) |
CT scan reveals hepatomegaly and bilateral pulmonary micronodules with hilar and mediastinal adenopathy.
A liver biopsy is performed.
Liver Core Biopsy - Histology Findings
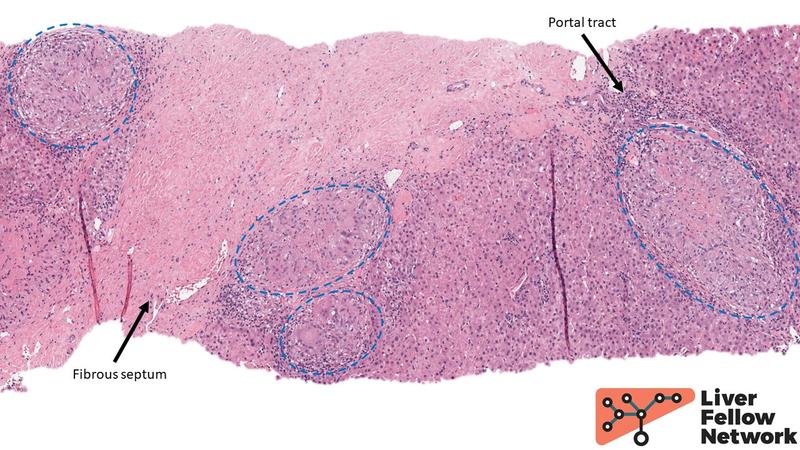
At low power, portal and periportal areas are notable for numerous well formed collections of epithelioid histiocytes – or epithelioid granulomas – with admixed multinucleated giant cells and lymphocytes (Fig. 1). The hepatic lobules reveal patchy inflammation as well.
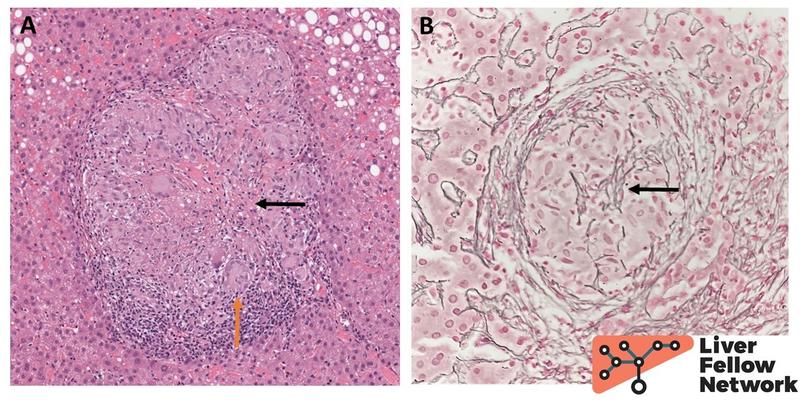
On higher power, the granulomas occasionally have a discrete bright pink mix of dead cells and fibrin which constitutes a special type of necrosis called fibrinoid necrosis (Fig. 2A). However, there is no evidence of “dirty-appearing” cellular and nuclear debris as seen in caseating type necrotizing granulomas. Fragments of reticulin fibers are noted within the granuloma as well (Fig. 2B).
While a diagnosis of exclusion, the histologic findings in light of patient’s history are consistent with hepatic sarcoidosis.
Hepatic Sarcoidosis
Sarcoidosis is a systemic granulomatous disease of unknown etiology that most commonly affects lungs and lymph nodes, followed by liver. In North America, it’s incidence is estimated at 7-to-11 per 100,000, affecting Black individuals and females 2-3 times as frequently. The patients typically come to medical attention in young-to-middle adulthood with respiratory symptoms (cough, difficulty breathing, chest pain) accompanied by radiologic abnormalities (pulmonary opacities, bilateral hilar lymphadenopathy), joint pain, skin and ocular lesions. Although hepatic involvement is present in most affected individuals, the patients are usually asymptomatic with only clues to diagnosis being abnormal liver function tests or hepatomegaly. Even with a classical clinical presentation, the diagnosis of sarcoidosis remains a diagnosis of exclusion which requires a careful consideration of alternative etiologies, a thorough clinical work up and an integration of clinicopathological findings.
The histologic hallmark of sarcoidosis is the presence of numerous granulomas in the affected tissue. The sarcoid granulomas are classically non-necrotizing epithelioid granulomas – that is well defined collections of large epithelioid macrophages variably surrounded by lymphocytes and other inflammatory cells. They are commonly found in portal or periportal locations and may contain a variable number of multinucleated giant cells. The granulomas often contain abundant reticulin fibers that can be highlighted by special stains. Older lesions progress to dense fibrous scars that can coalesce and occasionally mimic a mass lesion. Rarely, the sarcoid granulomas may contain central fibrinoid necrosis, but one should not see the abundant nuclear and cellular debris typical of infectious granulomas. The cytoplasmic inclusions such as Asteroid and Schaumann bodies (see examples here and here) are rarely found and are not specific to the diagnosis of sarcoidosis. In addition to granulomas, lobular hepatitis can be seen as well as variable degrees of fibrosis. Portal granulomatous venulitis with portal vein loss can occasionally occur, leading to portal hypertension. Focal bile duct injury and, less frequently, progressive chronic cholestatic disease with ductopenia can also be seen in sarcoidosis patients, which makes the distinction from primary biliary cholangitis and primary sclerosing cholangitis particularly difficult. Serology results and radiographic findings can be helpful in resolving this diagnostic dilemma.
Differential Diagnosis of Granulomatous Liver Disease
By definition, a granuloma is a collection of macrophages with or without other inflammatory cells that most commonly occurs in response to a chronic antigen exposure. In the liver, the presence of granulomas has a vast differential. However, the most common underlying etiologies in North America are infections, drug-induced-liver injury, primary biliary cholangitis, and sarcoidosis. Careful consideration of the specific morphologic features of granulomas and their location within the hepatic compartments can be helpful in narrowing down the differential diagnosis.
Infections
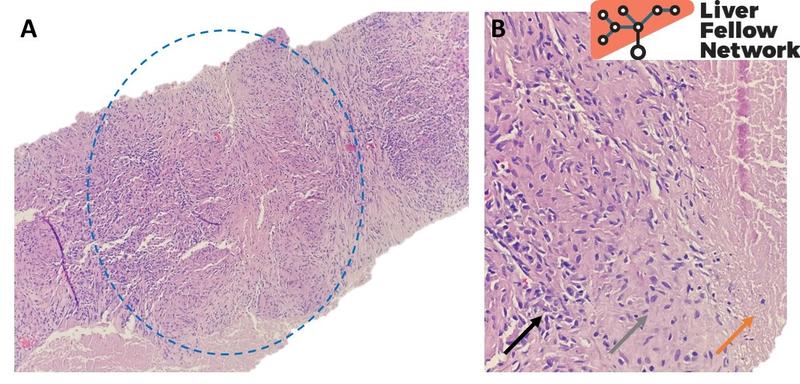
A variety of infectious agents are associated with a granulomatous hepatic response. More commonly seen bacterial culprits include Mycobacteria (Mycobacterium tuberculosis, M. avium-intracellulare and M. leprae), Bartonella henselae (Cat scratch disease), Brucella species and Rickettsia species. The resulting granulomas are classically necrotizing or “caseating”, that is they are composed of histiocytes palisading around a necrotic core of cellular debris, often surrounded by lymphocytes at the periphery (Fig. 3). However, epithelioid granulomas may also be seen in an infectious context. Special stains such as acid-fast stain for mycobacteria or silver stains for B. henselae can be used to confirm the presence of these microorganisms. However, these agents are difficult to identify in pathology specimens and negative stains do not exclude an infectious etiology. Cultures, PCR-based detection methods and serology are often more useful in identifying the specific organism.
Most common fungal agents include Histoplasma species, particularly in the endemic areas of lower Mississippi and Ohio River valleys, and Cryptococcus neoformans in immunocompromised patients. Special stains such as Gomori methenamine-silver (GMS) and periodic acid-schiff (PAS) can highlight fungal organisms.
Main parasitic etiologies to consider are Schistosoma species, particularly in patients with a history of travel to Southeast Asia, the Caribbean, the Middle East and tropical Africa regions, and Leishmania species causing visceral leishmaniasis, endemic in Southern Europe, the Middle East, Asia, China, South America and Africa, and seen in immunocompromised patients. These agents can be identified on H&E in forms of extracellular Schistosoma eggs or Leishmania amastigotes in Kupffer cells and macrophages.
Finally, certain viral hepatitides can uncommonly present with granulomas, including hepatitis viruses A, B and C, Cytomegalovirus and Epstein-Barr virus. Specific immunostains can help confirm the diagnosis.
Drug-induced-liver injury (DILI)
Lobular and portal granulomas can be seen in association with a wide variety of medications, the most common culprits being allopurinol, sulfonamides, isoniazid, phenytoin, hydralazine and quinidine. The granulomas in DILI are more commonly poorly defined histiocytic aggregates admixed with other inflammatory cells as opposed to the well defined epithelioid granulomas seen in sarcoidosis.
Biliary diseases of the liver
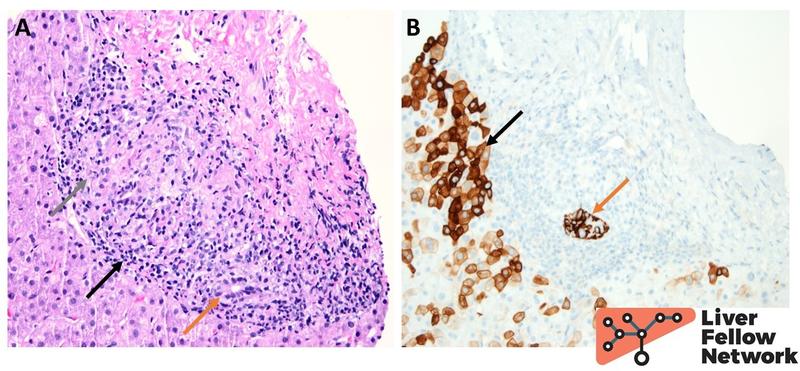
Poorly formed epithelioid granulomas are the characteristic histologic findings in primary biliary cholangitis, where they can be seen within the lobular parenchyma or in portal tracts, forming the classical florid duct lesions with native bile ducts (Fig. 4). Rarely, small epithelioid granulomas can also be seen in primary sclerosing cholangitis in response to bile extravasation. More details on these conditions can be found in Pathology Pearls on Primary Biliary Cholangitis and Primary Sclerosing Cholangitis.
Other causes
A variety of systemic disorders may manifest as hepatic granulomas. These include inflammatory bowel disease (both Crohn’s disease and ulcerative colitis), common variable immunodeficiency, and chronic granulomatous diseases as well as collagen vascular disorders. Another important etiology to consider in the appropriate clinical context is a nearby neoplasm. Primary tumors such as hepatocellular adenomas and carcinomas, hematolymphoid neoplasms such as Hodgkin lymphoma and a metastatic disease may all induce a granulomatous response in the surrounding liver. Finally, up to a third of all cases of hepatic granulomas remain idiopathic after a full clinical workup.
References
- Torbenson, Surgical Pathology of the Liver. 2018.
- Burt, MacSween’s Pathology of the Liver: Seventh Edition. 2017.
- Ganeshan D, Menias CO, Lubner MG, et al. Sarcoidosis from Head to Toe: What the Radiologist Needs to Know. Radiographics. 2018;38(4):1180-1200.
- Arkema EV, Cozier YC. Sarcoidosis epidemiology: recent estimates of incidence, prevalence and risk factors. Curr Opin Pulm Med. 2020;26(5):527-534.
- Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America. Analysis Based on Health Care Use. Ann Am Thorac Soc. 2016;13(8):1244-52.
Acknowledgements
Special thanks to Dr. Olga Aleynikova and Dr. David Escobar for providing images for the illustrative cases.