Pathology Pearls: Hepatocellular adenoma
Brief Case Presentation
A 64 year old male with history of hypertriglyceridemia presented with acute abdominal pain. Lab tests were obtained (below) and MRI abdomen showed a 4.0 cm mass.
Selected lab values are as follows:
A computed tomography guided liver biopsy was subsequently performed.
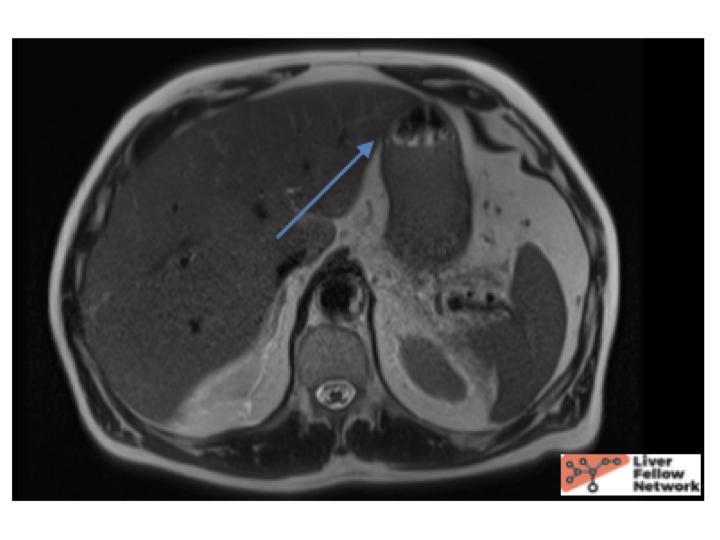
Liver biopsy findings
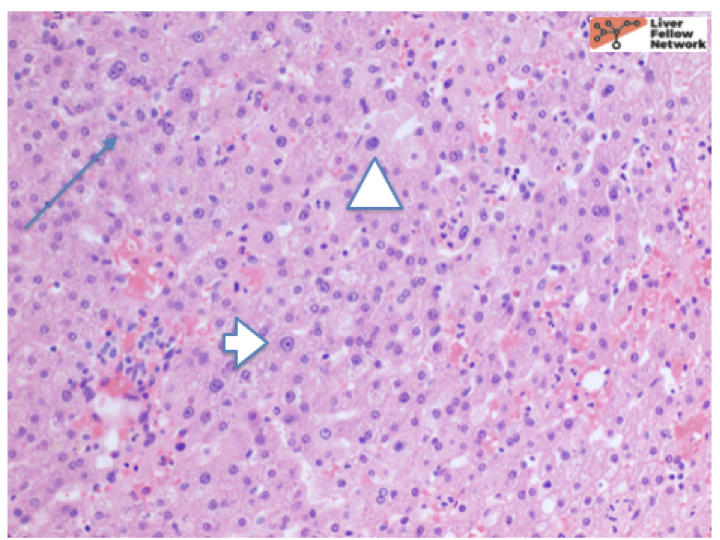
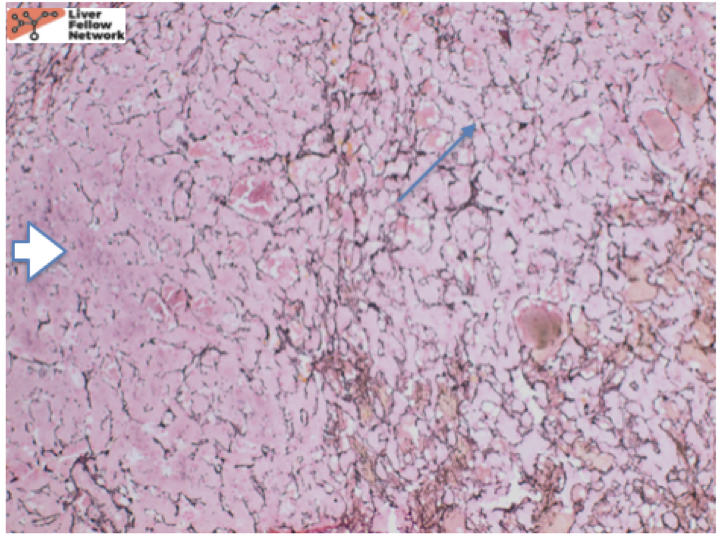
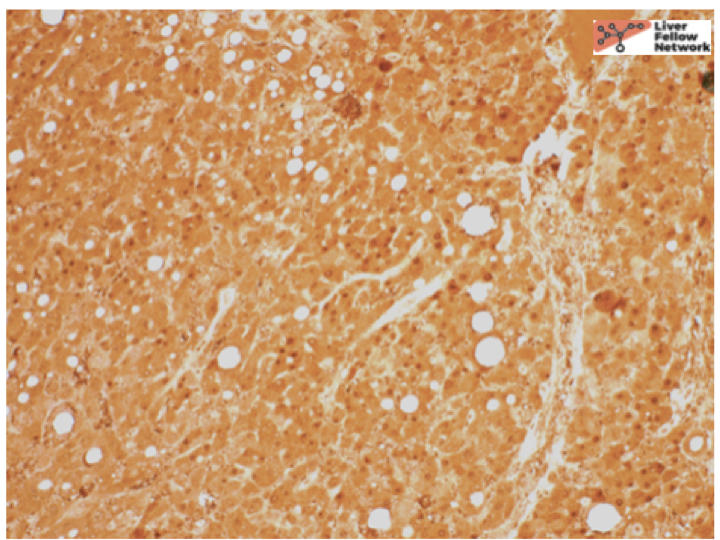
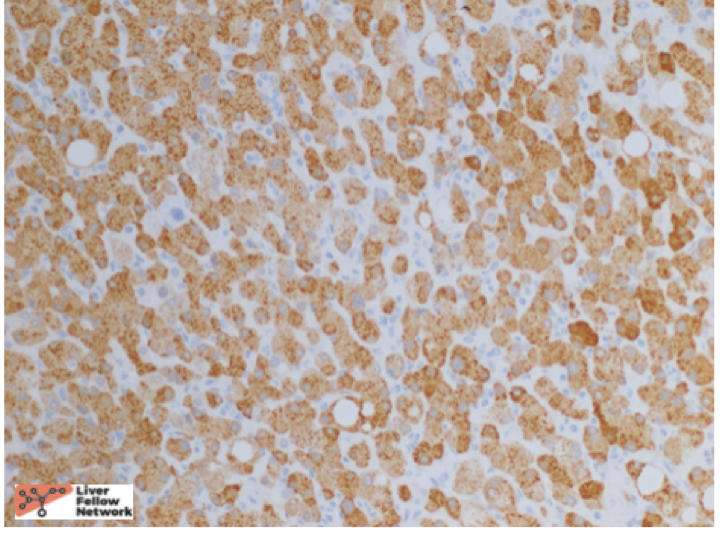
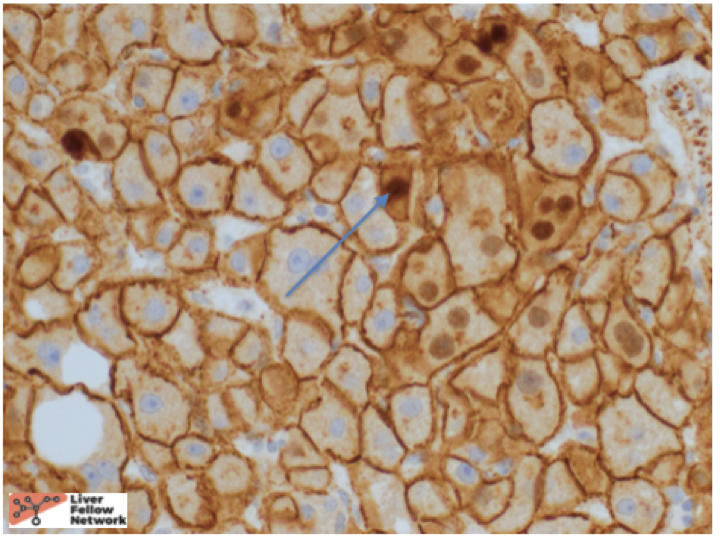
At low power, there is a well-circumscribed lesion with polygonal, eosinophilic cells and foci of steatosis (Figure 1). At medium power, there are unpaired arteries (absent interlobular bile ducts and portal veins) with thin hepatic plates (1-2 cell thick) and some multinucleated cells (Figure 2). There is no evidence of pseudo-acinar formation, vascular invasion, small cell change, or thickening of the hepatic plates (Figure 2 and 5). While there are rare areas that show some nuclear atypia (nuclear enlargement with prominent nucleoli) (Figure 4), and patchy chronic inflammation (Figure 3 and 4), there is no evidence of bile duct proliferation. Stains show intact expression of liver fatty acid binding protein (LFABP) (Figure 6). Glutamine-synthetase shows moderate and diffuse staining (Figure 7). Beta-catenin stain shows membranous expression with scattered loosely clustered foci of weak to moderate nuclear expression (Figure 8). Serum amyloid A (SAA) and C reactive protein (CRP) are negative.
The findings are most consistent with a beta-catenin-mutated hepatocellular adenoma.
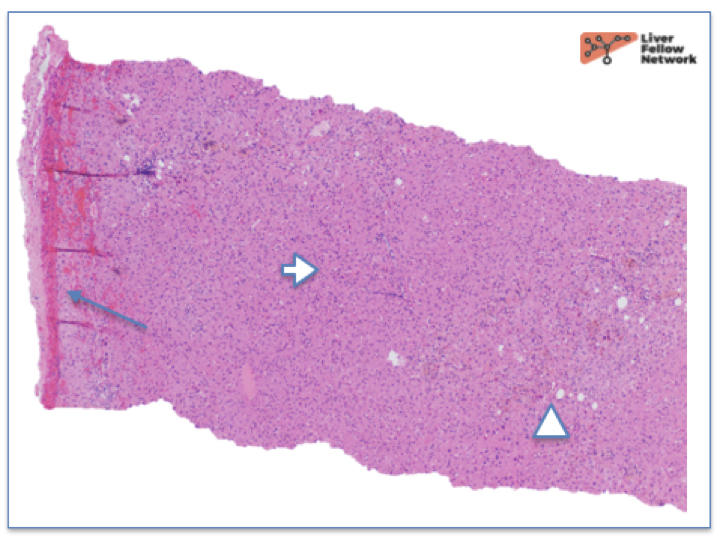
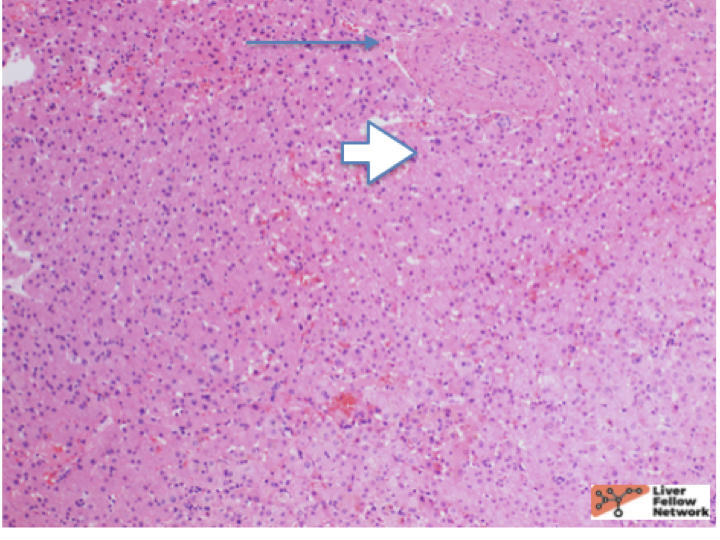
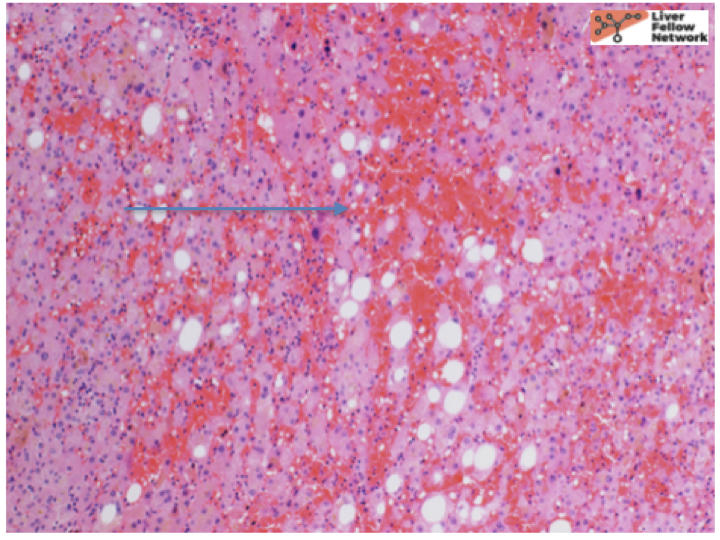
Hepatocellular Adenoma
Hepatocellular/Hepatic adenomas are benign neoplasms composed of phenotypically mature hepatocytes.
Most hepatic adenomas tend to occur in young to middle-aged women with exogenous estrogen exposure such as oral contraception or young to middle-aged women with fatty liver disease. The remaining either have no risk factors or have rare risk factors including excess androgen exposure (such as exogenous anabolic steroid use), genetic metabolic diseases such as glycogen storage diseases, McCune Albright syndrome or abnormal hepatic blood flow such as Abernathy syndrome or Budd–Chiari Syndrome. The term hepatic adenomatosis is used if there are 10 or greater hepatic adenomas.
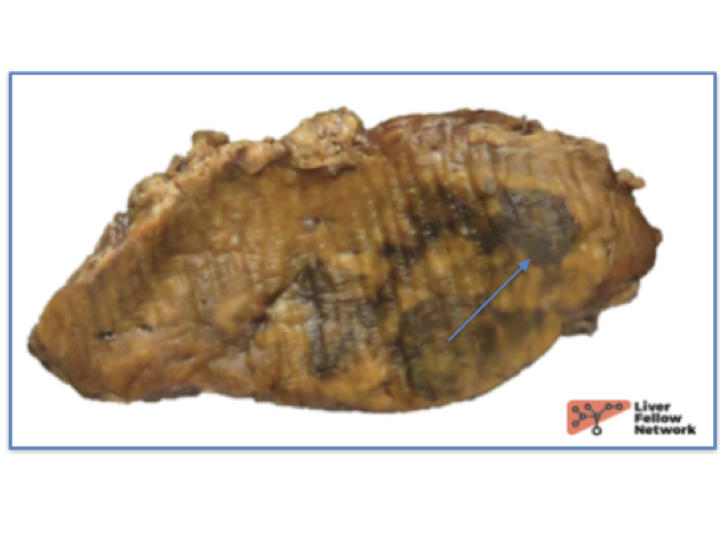
Grossly, hepatic adenomas appear as well circumscribed masses with areas of hemorrhage, necrosis or bile staining (Figure 10). They are often lighter in color compared to background liver.
Histologic features of Hepatocellular Adenoma
Hepatic adenomas develop in histologically normal, steatotic, and noncirrhotic livers.
Histologically, hepatic adenomas are distinct nodules of mature hepatocytes with or without foci of steatosis and a lack of portal tracts along with unpaired arteries. The cytoplasm can be normal, clear, steatotic, or it can contain pigment such as bile/lipofuscin. They have little to no cytological atypia, although mild patchy large cell change is acceptable in inflammatory or androgen related adenomas. The individual tumor cells resemble the background liver. Other atypical findings include numerous Mallory bodies or hyaline bodies.
The proliferative index on Ki-67 immunostain is similar to nonneoplastic liver.
By molecular analysis, they are clonal and driven by mutations (fewer mutations than hepatocellular carcinomas).
In rare cases however, the histological findings do not completely fall into the category of hepatocellular adenoma or well differentiated hepatocellular carcinoma especially on biopsy specimens. These cases do not have definite architectural atypia but too much cytological atypia or there are equivocal reticulin loss areas or the Ki-67 index is higher compared to background liver. In these cases, the terms atypical hepatocellular neoplasm/ hepatocellular neoplasm of unknown malignant potential are used.
Differential Diagnosis and subtypes of Hepatocellular Adenoma
The subtypes of hepatic adenoma are as follows:
- Immunostain classification
- HNF1-alpha–inactivated hepatic adenoma
Occur in the setting of exogenous estrogen. HNF1A mutations—most mutations are sporadic but rare germline mutations can be found, also being associated with mature onset diabetes of the young. Histologically often show macrovesicular steatosis and LFABP staining is lost.
- Inflammatory hepatic adenoma
Occur in exogenous estrogen settings or fatty liver disease. JAK/STAT pathway activation via mutations in IL6ST, STAT3, FRK, JAK1, or GNAS is seen. Faux portal tracts along with congestion and inflammation are observed. They are positive for CRP/SAA and LFABP staining is retained. Subset of inflammatory hepatic adenomas can demonstrate beta-catenin mutations.
- Beta-catenin–activated hepatic adenoma
There are no genetic risk factors and the morphology is non-distinct. CTNNB1 (beta-catenin) mutations are seen along with immunohistochemical staining of nuclear beta-catenin and/or diffuse glutamine synthetase (GS). Exon 3 (other than S45) mutations give a diffuse homogenous GS staining and often nuclear beta-catenin while exon 3 S45 give a diffuse heterogenous GS with little or no nuclear beta-catenin; and exon 7/8 mutations give a GS positive border and diffuse CD34 (except the border).
- Unclassified hepatic adenoma
Morphologically show mild steatosis and mild glycogen accumulation but overall they do not have any distinctive findings.
- Rare subtypes-High risk types of adenomas with malignant transformation
- Androgen-related hepatic adenoma
Occur in the setting of exogenous androgen. Histologically, they are frequently cholestatic, have small pseudo-glands with patchy but significant cytological atypia/large cell change. Immunohistochemically can coincide with HNF1-alpha inactivated, inflammatory, unclassified, or beta-catenin activated (majority).
- Pigmented hepatic adenoma
These have moderate to heavy lipofuscin within the tumor cells. Immunohistochemically, can also be classified as HNF1-alpha–inactivated or inflammatory adenomas. They are also likely to have cytological atypia and beta-catenin activation.
- Myxoid hepatic adenoma
These display sticky, tacky, mucoid feel by gross examination along with distinctive imaging findings, striking myxoid material (weakly Alcian blue positive, weakly mucicarmine positive) dissecting through the tumor trabecula histologically with some cases having a hepatic adenomatosis. LFABP loss and HNF1A mutations are possible. Associations with Carney complex are described in literature.
- Provisional: hedgehog-activated hepatic adenomas and hepatic adenomas positive for argininosuccinate synthase
Malignant transformation of Hepatocellular Adenoma
The risk factors for malignant transformation of hepatic adenomas include:
- Male gender
- Interval growth during follow-up by MRI
- Size >5 cm
- Beta-catenin activation
- Androgen as the underlying risk factor
- Pigmented adenoma
- Myxoid adenoma
Hepatocellular adenomas versus Hepatocellular carcinoma
Adenomas should have no architectural atypia, with no small cell change, nodule-within-nodule growth, and no thickened hepatic plates. The exceptions include pseudorosettes in androgen-related adenomas and physiological reduction in reticulin in areas of fatty change.
In summary, hepatocellular adenomas are benign neoplasms of mature hepatocytes arising in non-cirrhotic livers similar to the above case. They classically arise in high estrogen states with no histological atypia but when they do arise in other settings such as the above-mentioned case, they can have cytologic atypia. In such cases, it is important to distinguish them from well-differentiated hepatocellular carcinomas. Reticulin stain helps to show intact hepatic plates in hepatocellular adenomas (Figure 5). Intact LFABP staining helps to distinguish all hepatocellular adenomas from HNF1-alpha subtype adenoma (Figure 6). Diffuse Glutamine synthetase staining (Figure 8) and nuclear beta catenin staining (Figure 9) helps support the diagnosis of a beta-catenin mutated adenoma similar to our case.
References
- Torbenson, Atlas of Liver Pathology: A Pattern-Based Approach. 2019
- Choi W, Kakar S. Atypical Hepatocellular Neoplasms: Review of Clinical, Morophologic, Immunohistochemical, Molecular and Cytogenetic Features. Advanced Anatomical Pathology. 2018.
- Burt, MacSween’s Pathology of the Liver: Seventh Edition. 2017.
- Mounajjed T, Chandan VS, Torbenson MS, eds. Surgical Pathology of Liver Tumors. Switzerland: Springer Int Pub; 2015.
- WHO Classification of Tumors of the Digestive System, 5th ed. Lyon: IARC Press; 2019.
