Pathology Pearls: Immune Checkpoint Inhibitor Hepatitis
Brief Case Presentation
A 50-year-old patient with history of widely metastatic melanoma presents with fatigue, nausea, and vomiting. Scleral icterus is noted on physical exam. The patient has completed chemotherapy and finished a course of nivolumab approximately 10 weeks ago. After obtaining the following results from liver function tests, microbiology labs, and serologic studies, an MRCP is performed which revealed no abnormalities of the intra- or extra-hepatic biliary tree.
| Lab Test | Result |
|---|---|
| AST | 640 U/L (0 – 39 U/L) |
| ALT | 700 U/L (0 – 52 U/L) |
| Alk. Phos. | 600 U/L (34 – 104) U/L |
| GGT | 420 U/L (5 – 40 U/L) |
| Total Bilirubin | 5.9 g/dL (0.0 – 1.0 g/dL) |
| Albumin | 2.6 g/dL (3.5 – 5.7 g/dL) |
| Platelets | 30 K/uL (140 – 390 K/uL) |
| HAV | Negative |
| HBV | Negative |
| HCV | Negative |
| CMV | Negative |
| EBV | Negative |
| ANA | Negative |
| SMA | Negative |
| AMA | Negative |
Liver Biopsy
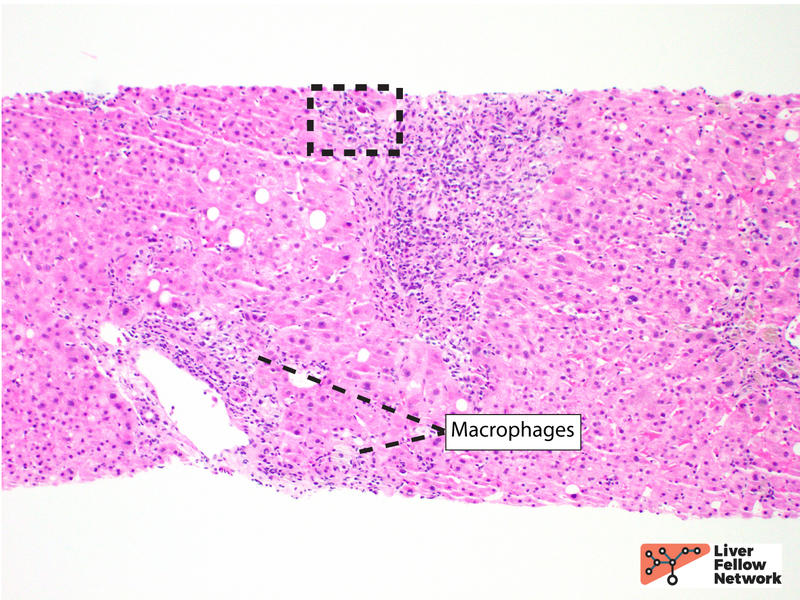
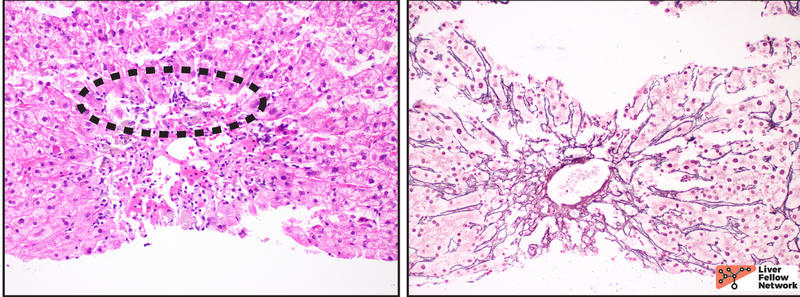
At scanning magnification, the core needle liver biopsy shows moderate portal inflammation and focal interface activity (Figure 1).
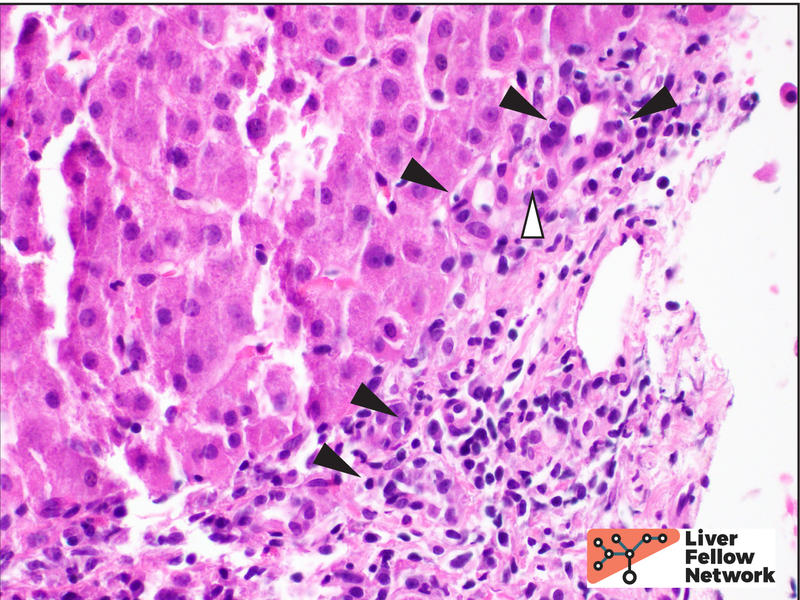
Higher magnification demonstrates a mixed portal inflammation (lymphocytes, occasional plasma cells, and few eosinophils and neutrophils) with focal interface activity. Some portal tracts also show an infiltrate of macrophages (also called histiocytes). The bile ducts are infiltrated by lymphocytes (lymphocytic cholangitis), causing eosinophilic change and vacuolization (bile duct damage) (Figure 2).
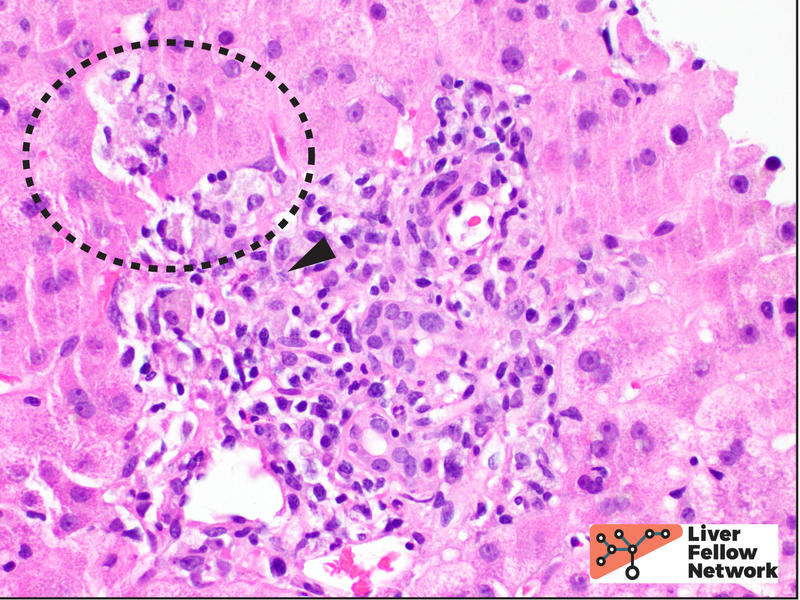
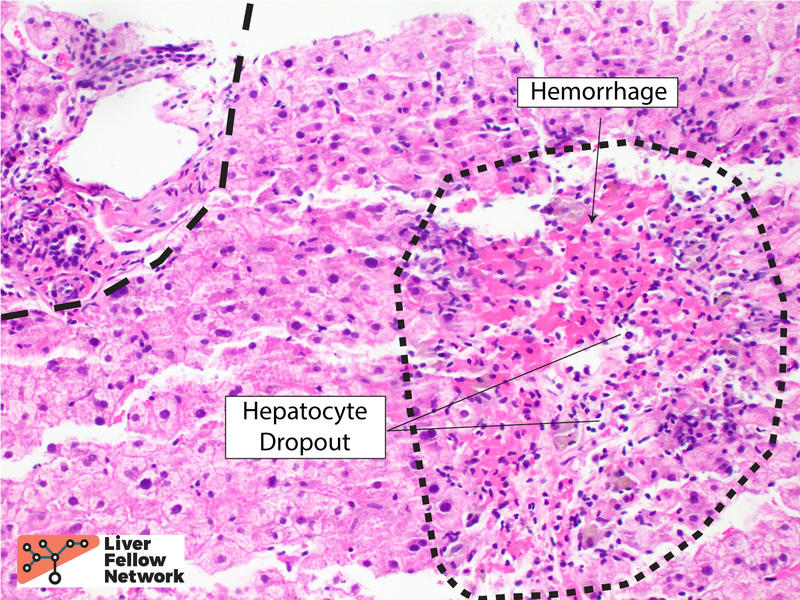
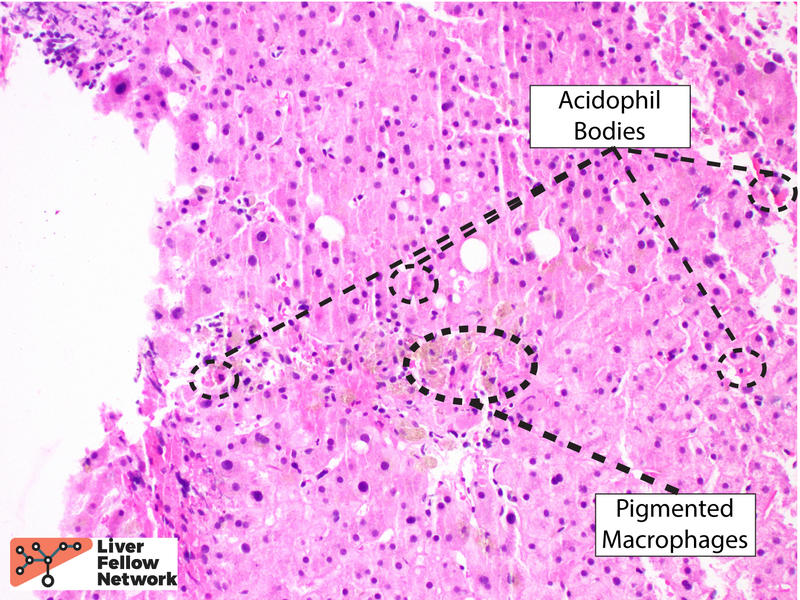
The lobular inflammation is predominantly comprised of lymphocytes and histiocytes in a centrilobular pattern and associated with hepatocyte injury, lobular disarray, hepatocyte necrosis (with occasional acidophil bodies), lipofuscin-laden macrophages, and hepatocellular cholestasis (Figures 3, 4 and 5).
In the clinical context provided, these findings may be compatible with immune checkpoint inhibitor (ICI) hepatitis.
Immune Checkpoint Inhibitor (ICI) Hepatitis
Immune checkpoint inhibitor (ICI) therapy has revolutionized the approach to aggressive, widely metastatic malignancies. Anti-CTLA4 and anti-PD-L1 agents have been approved for a wide variety of high-grade malignancies. However, ICI's are associated with a wide range of immune-related adverse events that can occur in any organ system; the frequency of these events is now becoming well known as the use of ICI therapy grows. Adverse events in the gastrointestinal tract and hepatobiliary system are common, however the spectrum of gastrointestinal symptoms is broad. The most common presenting symptom is diarrhea, followed by evidence of mucositis and hepatitis.
Liver injury occurs in 2-10% of patients on ICIs. Average onset may be approximately 4 - 12 weeks for anti-PD/PD-L1 agents and approximately 3 - 9 weeks for anti-CTLA4 agents. Injury is typically hepatocellular (elevated transaminases) or biliary (elevated alkaline phosphatase or gamma-glutamyl transpeptidase, GGT).
The histologic features of ICI are only recently being described, and are a subject of ongoing investigation. At the time of writing this Liver Fellow's Network post, two major histologic patterns have been described:
- Panlobular Hepatitis ("Hepatitic Pattern"): The most common type of injury (~50% of cases) shows predominantly lobular inflammation (lymphocytes and macrophages) which may be more centrilobular with lobular disarray, and milder portal inflammation. Features of this type of injury were the predominantly finding in the case described above.
- Cholangitic Pattern: The second most common type of injury (~30% of cases) shows predominantly portal-based inflammation (lymphocytes, occasional plasma cells, neutrophils, and eosinophils) with associated bile duct injury. Features of this type of injury were also observed in the case described above.
- Mixed Hepatitic/Cholangitic: A mixed pattern may be seen in 10-15% of cases.
There are other, rarer, patterns of injury described in case reports and case series. Some reports have described lobular microgranulomas associated with ICI's, and these may be associated with CTLA-4 therapy. Rare reports have described steatosis and steatohepatitis histologically identical to Non-alcoholic steatohepatitis (NASH), which resolved on subsequent liver biopsies after discontinuation of ICI therapy. Also, a pattern of nodular regenerative hyperplasia has rarely been reported in the setting of anti-PD/PD-L1 therapy.
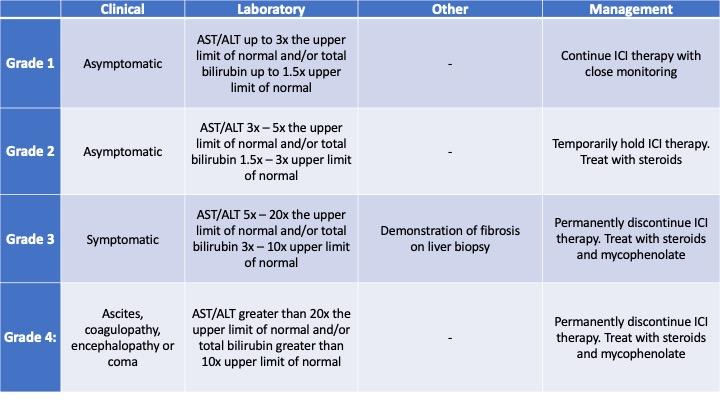
The American Society of Clinical Oncology (ASCO) recommends grading the severity of ICI hepatitis using the criteria in Table 1, below.
Differential Diagnosis
The histopathologic differential diagnosis fo ICI hepatitis is broad. Due to significant histologic overlap, autoimmune hepatitis, drug-induced liver injury, and acute viral hepatitis are all worthy consideration. Fortunately, these etiologies may be ruled out with immune serology studies, microbiology lab results, and a careful drug history. Additional differential diagnoses for the cholangitic pattern include biliary obstruction and primary biliary cholangitis. Lastly, ICI hepatitis may mimic acute cellular rejection in liver transplant patients.
References
- Patil, P. A. & Zhang, X. Pathologic Manifestations of Gastrointestinal and Hepatobiliary Injury in Immune Checkpoint Inhibitor Therapy. Arch Pathol Lab Med 145, 571–582 (2020).
- Zhang, M. L. & Deshpande, V. Histopathology of Gastrointestinal Immune-related Adverse Events: A Practical Review for the Practicing Pathologist. Am J Surg Pathol Publish Ahead of Print, (2021).
- Brahmer, J. R. et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 36, JCO.2017.77.638 (2018).