Deciphering the code of iron overload
What is hereditary hemochromatosis?
Hereditary hemochromatosis (HH) is an inherited autosomal recessive iron overload disorder which can lead to excessive absorption of iron. Mutations in the HFE gene are seen in the majority of individuals with HH, but other mutations do exist (i.e. HJV, HAMP, TFR2). The two most common HFE mutations are C282Y and H63D. Homozygosity for C282Y is more commonly seen in patients with clinical disease.
Need a refresher on the pathophysiology of iron absorption? ACG’s 2019 Clinical Guideline on Hereditary Hemochromatosis has a great review!
Who should undergo genetic testing?
Due to variable prevalence of the C282Y and incomplete penetrance of the mutation, ACG, AASLD, and EASL recommend against screening the general population. However, HFE testing should be performed in first degree relatives of patients diagnosed with HH.
HH could also be considered in patients with porphyria cutanea tarda, well defined chondrocalcinosis, hepatocellular carcinoma, and type 1 diabetes and in those with abnormal iron studies.
Patients with unexplained chronic liver disease should also be evaluated. However, it is important to recognize that secondary iron overload may be related to underlying liver disease rather than HH.
Don’t confuse HH with secondary iron overload!
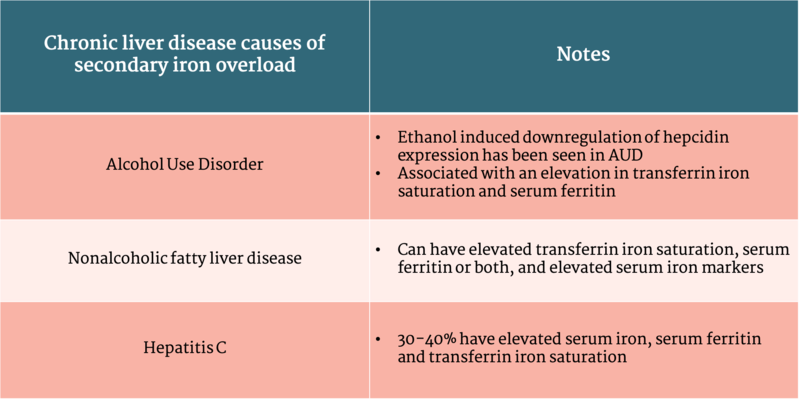
Secondary iron overload causes excess absorption and organ deposition of iron, but is unrelated to one of the genetic mutations leading to HH. This can be due to iron loading anemias, parenteral iron use, other liver diseases, malignancy, or chronic inflammatory states (Table 1).
On histology, secondary iron overload generally causes iron deposition in Kupffer cells whereas hemochromatosis leads to iron deposition in the hepatocytes
Table 1: Chronic liver disease causes of secondary iron overload
Table adapted from ACG Clinical Guideline: Hereditary Hemochromatosis
How should the diagnosis of HH be made?
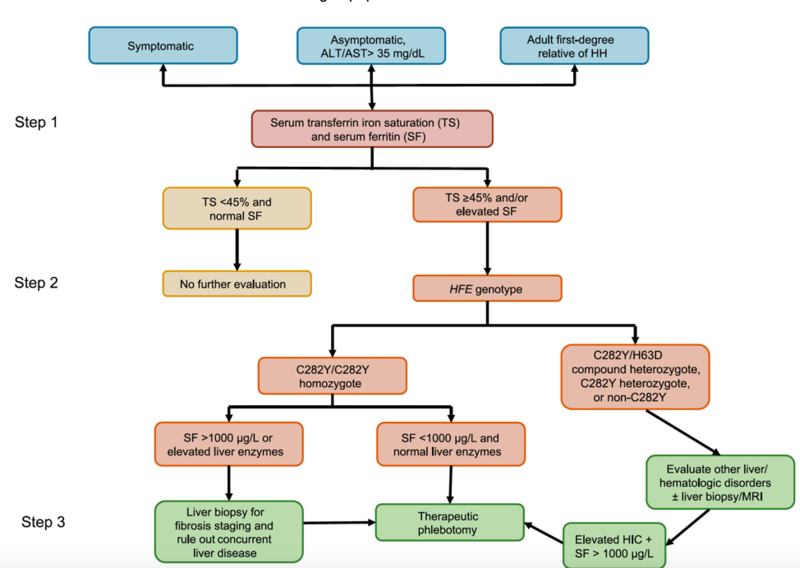
Iron studies including transferrin saturation and serum ferritin should be the initial test to determine if iron overload is present. Although variable cutoffs exist, AASLD recommends a transferrin saturation cutoff of 45%, which identifies 97.9-100% of C282Y homozygotes.
Ferritin is a circulating iron storage protein that is made in the liver. While an elevated ferritin level can indicate excess iron stores, it is important to remember that it’s also an acute phase reactant and thus has a significant false positive rate, particularly in patients with necroinflammatory liver disease (alcoholic liver disease, chronic hepatitis B and C, non-alcoholic fatty liver disease), lymphomas, and patients with other non-hepatic chronic inflammatory conditions.
Figure 1: Diagnostic and treatment algorithm for HH
Image taken from ACG Clinical Guideline: Hereditary Hemochromatosis
With the advent of HFE genetic testing, a liver biopsy is not routinely used to establish the diagnosis of HH. AASLD recommends using a liver biopsy only to determine the presence or absence of advanced fibrosis. Identifying patients with cirrhosis can have prognostic value and can lead to changes in clinical practice such as screening for hepatocellular carcinoma and esophageal varices.
Serum ferritin can also help identify patients who are at increased risk of cirrhosis. There is a 20-45% prevalence of cirrhosis in C282Y homozygotes with a serum ferritin >1000.
A liver biopsy in C282Y homozygotes with a serum ferritin <1000 ng/mL is not recommended unless there are concurrent risk factor for cirrhosis. This is because in the absence of risk factors, <2% of C282Y homozygotes with a serum ferritin <1000 ng/mL have advanced fibrosis or cirrhosis.
When should treatment be initiated?
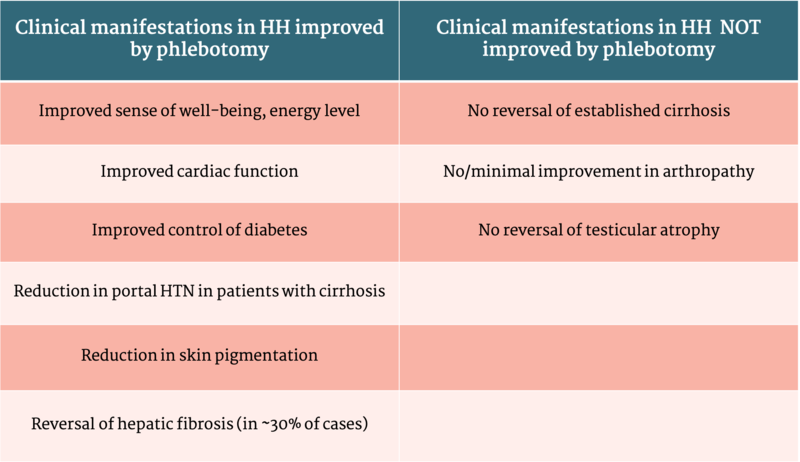
Table 2: Effect of phlebotomy on clinical manifestations in HH
Table adapted from Diagnosis and Management of Hemochromatosis: 2011 Practice guideline by the American Association for the Study of Liver Disease
Treatment should be initiated in C282Y homozygotes with a serum ferritin >300 ng/mL in men and >200 ng/mL in women along with a transferrin saturation of ³45%. In contrast, homozygous patients with a normal serum ferritin can be closely monitored with serial liver aminotransferases and ferritin levels, as they are unlikely to develop significant iron overload later in life.
The initial phase of phlebotomy involves weekly removal of 500 mL of blood, but larger or smaller amounts can be removed based on needs and tolerance of patient. Hemoglobin levels should be checked before and during treatment to ensure it is above 11 g/dL.
The goal of phlebotomy is to reduce the serum ferritin to a goal of 50-100 ng/mL. Once this is achieved, maintenance phlebotomy can be initiated, occurring approximately 3 to 4 times per year.