Dyspnea After Liver Transplant: What’s Taking His Breath Away?
Symptoms and Diagnosis of PTLD
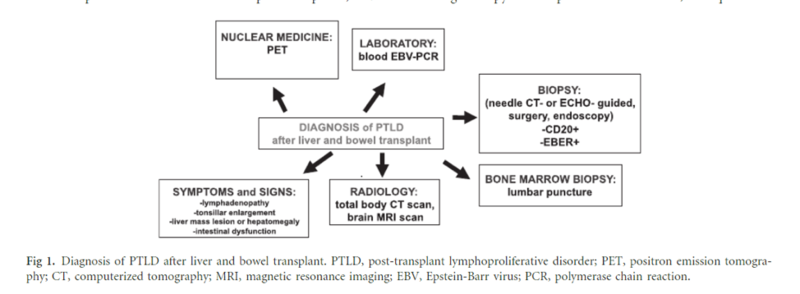
The clinical presentation of PTLD is highly variable. Due to the systemic nature of the disease, a high index of suspicion must be maintained when considering PTLD in a patient’s differential diagnosis. Patients may present with constitutional symptoms, such as weight loss, fevers or night sweats, and swollen lymph glands, or more specific complaints, including abdominal pain, gastrointestinal bleeding, or sore throat. Physical exam signs include the following: lymphadenopathy, hepatosplenomegaly, oral ulcers, and focal neurologic deficits. Histopathologic examination is ultimately necessary to confirm the diagnosis of PTLD. Remember, the incidence of PTLD after 5 years post liver transplant is 1.1%. This schematic outlines the important elements of PTLD diagnosis:
Figure 1 - Approach to Diagnosis of PTLD after Liver Transplant
Image taken from: Managing the Challenge of PTLD in Liver and Bowel Transplant Patients. BJH 2015; 169:157-172.
Risk Factors for PTLD
The biggest risk factors for PTLD are immunosuppression, EBV infection, and age. Both increased intensity and duration of immunosuppression increase the risk for formation of PTLD. Calcineurin inihibitors, anti-thymocyte globulin, and azathioprine are the agents with the greatest risk for causing PTLD.
PTLD is more than twenty times more common in EBV seronegative patients than EBV seropositive patients. Immunosuppression leads to T cell downregulation, which can promote EBV infection, lymphocyte proliferation, and ultimately PTLD.
Management of PTLD
The first step in treatment for PTLD is reduction in immunosuppression. Expert recommendations include stopping anti-proliferative agents such as azathioprine and mycophenolate and reducing calcineurin inhibitor therapy by 30-50%. Studies show a 45% response rate, 37% complete response rate, and 17% relapse rate with this strategy.
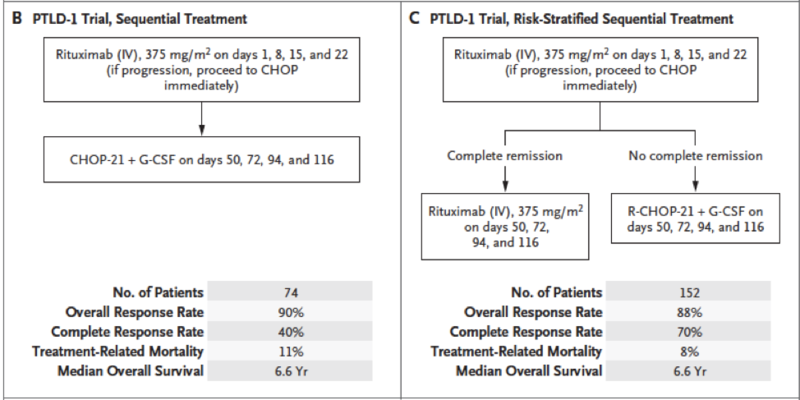
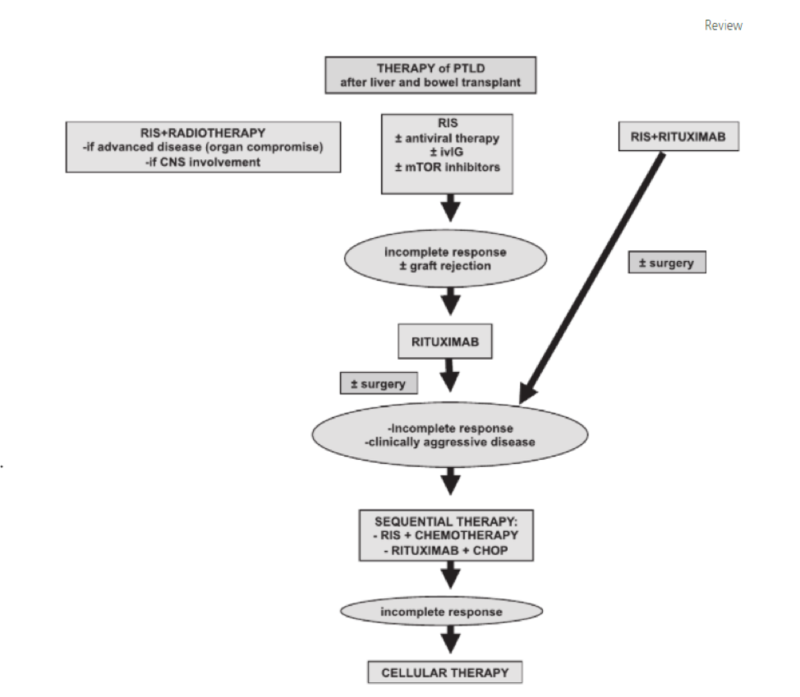
The gold standard treatment regimen is R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. Below are the summarized results of the PTLD-1 trial (Figure 2), which notably demonstrates a high response rate to R-CHOP with a median overall survival of 6.6 years, as well as an algorithm for PTLD treatment (Figure 3).
Figure 2 - R-CHOP treatment regimens in patients with PTLD from the PTLD-1 trial
Taken from: Post-transplant Lymphoproliferative Disorder in Adults. N Engl J Med 2018; 378:549-562. CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone; PTLD: post-transplant lymphoproliferative disorder
Figure 3 - Approach to Management of PTLD
Taken from: Managing the Challenge of PTLD in Liver and Bowel Transplant Patients. BJH 2015; 169:157-172.