How much liver is enough?
Living donor liver transplant: donor selection and donor-recipient pairing
1. The donor evaluation:
a. Living donor hepatectomy is a technically challenging, high stakes operation with associated risk. It is unique from other surgery in that it does not medically benefit the donor (in fact, it risks physical harm). The donor is a healthy person who does not have a disease, leaving little room for error. Therefore, any potential donor requires a comprehensive evaluation prior to surgical consideration. The evaluation begins with a detailed history and physical to evaluate for any preclusions to donation. Some important contraindications include chronic liver disease, malignancy, cardiovascular disease, certain lipid disorders (mild dyslipidemia that is diet-controlled or controlled with minimal medication might be acceptable), diabetes, BMI >30-35, coagulation disorders, drug use (marijuana use policies vary by institution), and alcohol abuse. Laboratory evaluation includes complete blood cell count, comprehensive metabolic panel, coagulation profile, lipid and endocrine profiles, liver disease screening (e.g., alpha 1 antitrypsin), viral serologies, and blood type. Some centers screen for hypercoagulable disorders. Cardiac assessment varies by donor age and risk factors but includes EKG and echocardiogram with consideration for CT coronary angiogram or cardiac stress test. Imaging studies include chest x-ray, triple-phase contrast CT abdomen and/or liver MRI/MRCP. A liver biopsy will be performed if indicated by medical history, abnormal blood tests or imaging (e.g., steatosis, iron deposition). The donor is evaluated by a living donor team that includes a transplant surgeon, hepatologist, social worker, psychologist, and independent living donor advocate. The potential donor’s evaluation and testing results are reviewed at a multi-disciplinary conference. The team must ensure that no contraindications to donation exist, that the potential donor understands the risks of the surgery, and that he or she is donating voluntarily without coercion. Donor safety is paramount.

2. Graft selection:
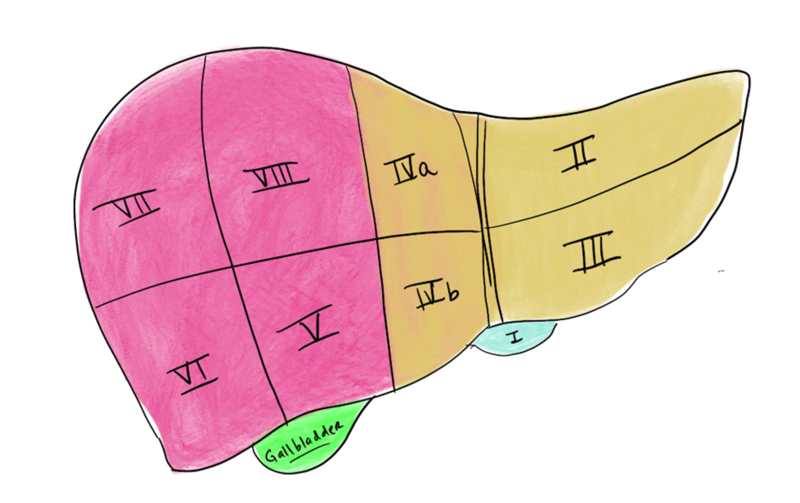
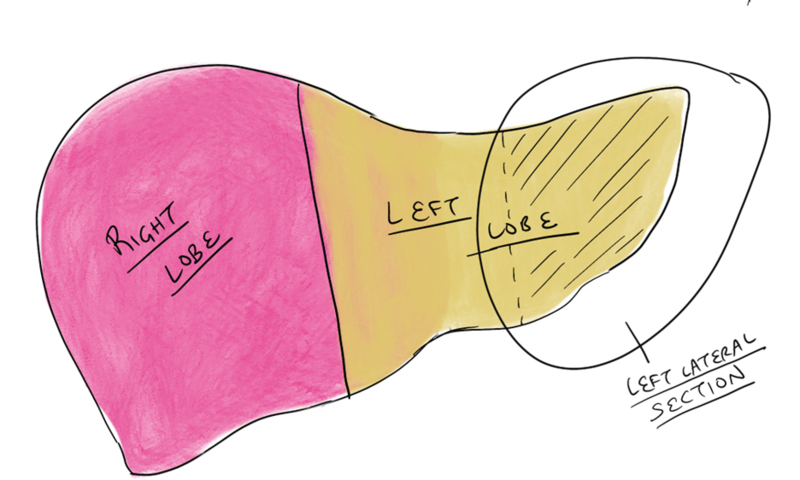
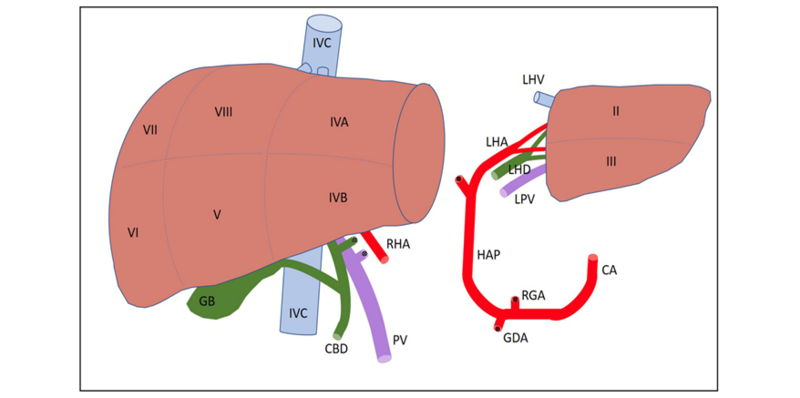
a. Anatomy: The liver is made up of eight segments (Figure 1). The left lateral section contains segments II and III and is usually allocated to a pediatric recipient (Figure 2). The right lobe is segments V to VIII and is usually allocated to an adult or adult-sized pediatric recipient (Figure 3). The left lobe is segments I to IV and is usually allocated to a small adult or child. (Note: the caudate lobe (segment I) might not be included with the left lobe graft.)
Figure 1- The segmental anatomy of the liver
Figure 2 – Possible graft options
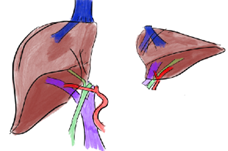
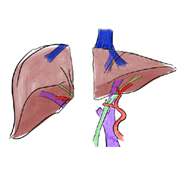
Figure 3: Depiction of a left donor hepatectomy and a right donor hepatectomy
b. Imaging: CT or MRI scans obtained during the evaluation process direct graft selection. They are used to evaluate liver size, parenchymal abnormalities, and anatomy. Parenchymal abnormalities include masses or steatosis. If concern for at least 10% steatosis on imaging, liver biopsy may be recommended. Other indications for liver biopsy include abnormal liver function tests, family history of liver disease, and/or positive genetic markers.
c. Graft size: Liver size is evaluated through volume analysis of cross-sectional imaging. Total liver size, predicted graft, and remnant liver volumes are determined. The right hepatic lobe is usually 55% to 80% of the donor’s total liver volume (TLV). Remnant liver volume should be greater than 30-35% of TLV to avoid post-operative liver failure. Adult-to-adult liver transplantation in the U.S. typically requires right lobe donation to provide sufficient volume for the recipient. For the recipient, the graft-to-recipient weight ratio (GRWR) is calculated (GRWR = predicted graft weight (kg)/recipient weight (kg) x 100%). A graft that provides greater than 0.8% of the body weight of the recipient is generally deemed safe. Some centers will accept GRWR 0.6%- 0.8% in a graft with perfect outflow for a recipient without significant portal hypertension. GRWR less than 0.6% significantly increases the risk for portal hyperperfusion, early allograft dysfunction, and small-for-size syndrome (SFSS).
d. Anatomic considerations: Most centers will use virtual three-dimensional models (reconstructed from cross-sectional imaging) to better visualize anatomy and understand the relationship of critical structures (Figure 4). Some anatomic variants increase either donor or recipient risk and might be considered prohibitive for donation.
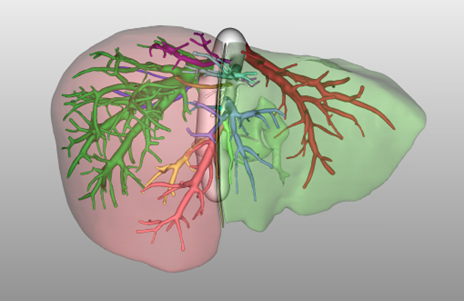
Figure 4. MeVis Liver Suite Software (MeVis Medical Solutions AG, Bremen, Germany) 3-Dimensional reconstruction of prospective donor liver. Right hepatic lobe is colored in red; left lobe is colored in green.
i. Hepatic artery considerations: Conventional anatomy consists of the proper hepatic artery branching into the left hepatic artery and the right hepatic artery with a segment IV branch originating from the left hepatic artery. A completely replaced right hepatic artery that arises from the superior mesenteric artery can be favorable as it provides a longer arterial length. Similarly, a replaced left hepatic artery arising from the left gastric artery might also have longer length than a conventional left artery. In contrast, accessory vessel anatomy might require back-table reconstruction or two arterial anastomoses during graft implantation. This can increase the technical difficulty and risk of hepatic artery thrombosis of the graft. Some centers consider the presence of two or more graft arteries to be a relative contraindication to donation. The need for multiple arterial reconstructions to a graft, especially with small diameter vessels, increases risk of thrombosis and biliary complications.
ii. Portal vein considerations: Conventional anatomy consists of a main portal vein bifurcating into a left and a right branch. Variations in portal vein anatomy are less common than in the arterial anatomy. When there is a trifurcation (7% of the population), a surgeon can perform a back-table reconstruction prior to graft implantation (Figure 5). Few potential donors have segmental branching which can result in 3 portal vein branches to a right lobe graft.
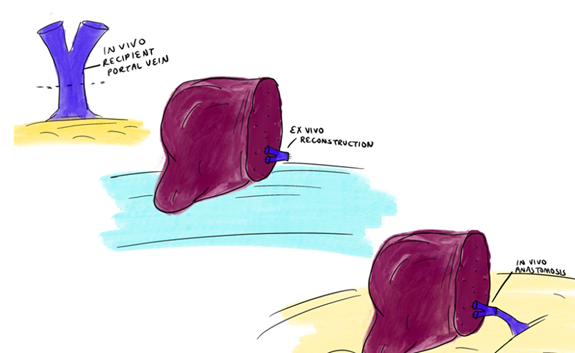
Figure 5: Recipient portal vein bifurcation resected on backbench after hepatectomy. A ‘Y-graft’ anastomosed to donor anterior and posterior portal vein on backbench results in 90 degree rotation in orientation.
iii. Hepatic vein considerations: The liver is drained by the left, middle, and right hepatic veins. The middle hepatic vein is a guide for the transection plane for left or right lobe hepatectomy. (In our institution, the middle hepatic vein stays with the left lobe.) When a right lobe graft does not include the MHV, there are often large segmental veins which require reconstruction to optimize venous outflow. Generally accepted indications for reconstruction are veins >5mm diameter or estimated outflow contribution of >10% (Figure 6). This is less common for left or left lateral section grafts.
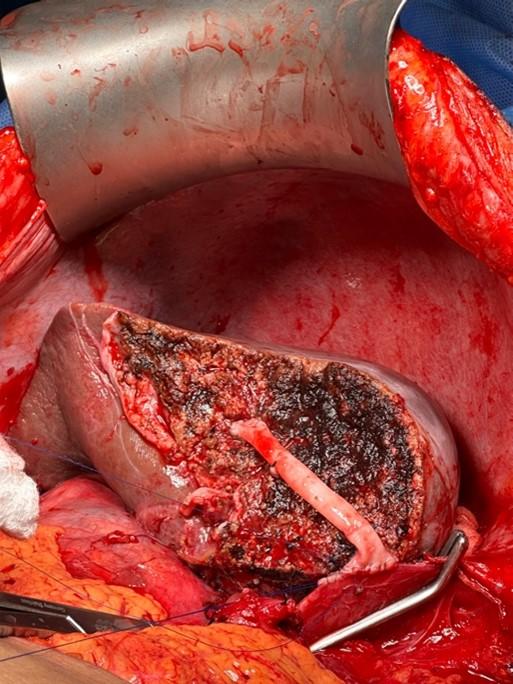
Figure 6: Venous reconstruction of a segment 5 vein in a living donor liver transplantation with a right lobe. (Courtesy of James Pomposelli, MD, PhD, University of Colorado)
iv. Bile duct considerations: Bile duct anatomy is highly variable. Only about half the population have ‘conventional’ anatomy that consists of a posterior sector duct coursing posterior to the right anterior duct before joining the right anterior duct. Often, variations require two bile duct anastomoses in the recipient (Figure 7). Due to the relatively high rate of biliary complications, the biliary anastomosis is often referred to as the ‘Achilles heel’ of living donor liver transplant – it is preferable to have one anastomosis that can be constructed in a standard duct-to-duct fashion. If two anastomoses are required, then reconstruction with Roux-en-Y hepaticojejunostomy might be necessary (Figure 8).
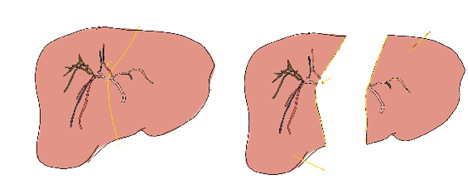
Figure 7 – 3D reconstruction of a donor liver’s biliary system demonstrated 2 bile ducts in the right lobe graft.
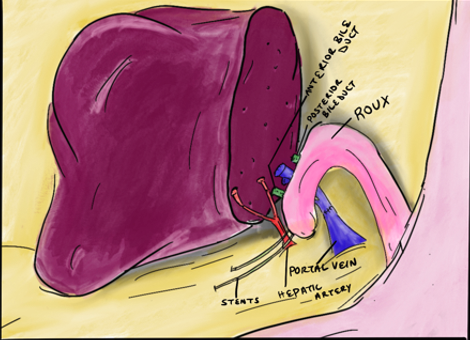
Figure 8 – Recipient reconstruction of two bile ducts with a roux-en-y hepaticojejunostomy
3. Recipient considerations:
a. Recipient selection: Recipient selection for the appropriate donor graft is based on several criteria. As discussed, a minimum predicted GRWR > 0.8% is recommended. Some also consider the graft volume relative to standard liver volume > 40% (calculated by estimated graft volume/recipient’s total liver volume x 100%). Right lobe grafts, being larger, frequently offer optimal graft volume for recipients with heavier weight, higher MELD scores (>20), age >45 years, and/or severe portal hypertension. There is increased risk of graft dysfunction with smaller grafts in this population. Other considerations include risk factors for a complicated hepatectomy such as prior abdominal surgery or history of spontaneous bacterial peritonitis. Left lobe grafts are used typically for children or smaller adults with lower MELD (<20) and mild portal hypertension.
b. Portal hypertension: Portal hyperperfusion of the graft can cause early allograft dysfunction and small for size syndrome. Portal inflow modulation is recommended for smaller grafts with portal vein pressure >20mmHg (some institutions perform inflow modulation for portal vein pressure >15 mmHg), portal flow greater than 2.5cc/gram graft weight, hepatic artery flow <100 mL/min, or poor arterial diastolic flow. Techniques for modulation include splenic artery ligation, porto-systemic shunting, or splenectomy.
4. Outcomes:
a. Donor risk: Donor outcomes are closely monitored and examined. Some of the more common risks include 1) bleeding (the need for transfusion can be reduced with intra-operative use of autologous blood recovery system (e.g. Cell Saver)); 2) infection; 3) venous thromboembolism; 4) incisional hernia; 5) biliary complications. There is a risk of remnant liver insufficiency (rare when remnant liver volume is > 30%). Overall donor morbidity is 8% to 40%. Mortality risk is 0.2-0.5%. Some studies have shown that right hepatectomy is associated with higher overall mortality and morbidity when compared with left hepatectomy for donation. Historically, right hepatectomies have higher rates of bile leak and stricture, cardiopulmonary complications, and hospital length of stay. Additionally, there are higher rates of postoperative hepatic dysfunction. Some recent studies including a meta-analysis, report no significant difference in donor complications between right and left hepatectomy. There have been no differences in adverse outcomes between surgical techniques (minimally invasive versus open surgery) when evaluating safety, overall morbidity, or mortality. In select donors, a minimally invasive approach potentially offers quicker recovery and reduced post-operative pain.
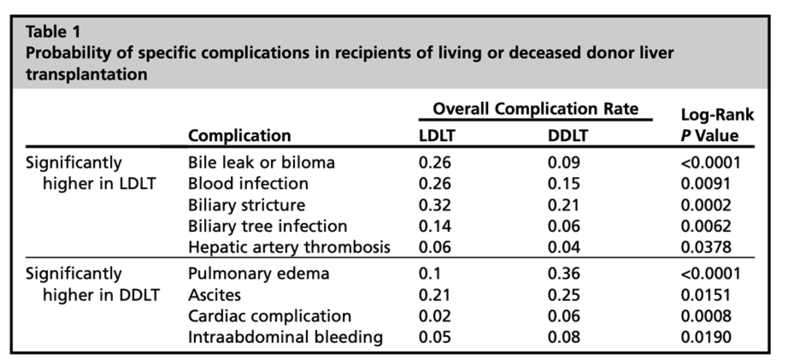
b. Recipient risk: Studies have demonstrated no difference in postoperative biliary or vascular complications between recipients receiving left lobe grafts compared with right lobe grafts. There are, logically, higher risks of SFSS with a left lobe which can be mitigated with appropriate donor and recipient selection. When compared with deceased donor whole liver allograft transplantation, living donor liver transplant has higher risks of vascular and biliary complications– hepatic artery thrombosis (6.5% vs. 2.3%) or portal vein thrombosis (2.9% vs. 0.0%) ,leak or stricture- (40% vs. 25%) (Figure 9). Other complications after living donor transplantation include but are not limited to infection (32%) and early allograft dysfunction (16%-19%).
Pediatric grafts into adult recipients
In general, pediatric grafts are preferentially allocated to pediatric recipients for optimal benefit to the pediatric recipient pool. Policy changes made in 2005 were implemented to help facilitate this by regionally sharing all pediatric liver grafts. In cases when pediatric grafts are turned down for all possible pediatric recipients, they can be successively offered to adults. A concern in this scenario is inadequate graft volume and the subsequent risk of SFSS.
- Graft selection: Use of pediatric grafts in adults requires special consideration. Usually, graft weight or volume ratios will guide selection. Some studies recommend a donor liver weight to estimated recipient liver weight ratio of >0.4 along with preservation times <12 hours to avoid complications. Others recommend selection criteria similar to living donor transplantation with a GRWR of >0.8%. Typically, pediatric grafts accepted for adult recipients have been turned down by a median of three pediatric centers, most commonly with refusal codes for donor size/weight. These grafts are usually larger, from DCD donors, and may have elevated transaminases compared to grafts accepted for pediatric recipients.
- Recipient selection: Due often to the complexity of the case and size mismatch with pediatric allografts, some centers recommend using ‘simple’ adult recipients - close to the hospital, with an anticipated ‘easy’ hepatectomy, no prior abdominal surgeries, and no portal vein thromboses to minimize cold ischemia time. Adult recipients of pediatric livers usually have lower BMIs and are more likely to be female. Recommended GRWR is > 0.8%, similar to that recommended for living donor liver transplantation. Pediatric grafts have decreased 90-day graft survival in adult recipients if GRWR <0.8%.
- Outcomes: Outcomes data for pediatric liver allografts is variable. Most studies demonstrate comparable graft survival and overall complications between pediatric-to-adult grafts and adult-to-adult grafts with appropriate recipient selection. There are increased risks of hepatic artery thrombosis (HAT), likely due to due to small size of the donor artery and/or the unavoidable arterial size discrepancy.
Split liver grafts
Split liver transplantation offers a solution to address organ shortages while also providing appropriately sized grafts for pediatric recipients (Figure 10)
- Donor selection: Ideally, donors should be healthy with a BMI <25 kg/m2, age <35 years old, and minimal comorbidities. Most often, the graft is split into a left lateral section and an extended right lobe. The left lateral graft (segments II and III) is allocated to a pediatric recipient and the extended right graft (segment I, IV-VIII) to an adult recipient. A normal sized liver allograft may also be split into left lobe (segments I-IV) and right lobe segments V-VIII. Sometimes the caudate lobe (segment I) remains with the right lobe. This type of split can allow transplantation of two adult recipients.
- Recipient selection: Similar to LDLT, GRWR should be considered. Of note, LDLT grafts are taken from very healthy donors under ideal conditions to minimize cold ischemia time. To account for deceased donor factors, a minimum GRWR > 1% is considered adequate for split liver transplantation. The average adult left lobe graft weight is 400-500g, which is usually sufficient for a recipient weighing 40-50kg. The average adult right lobe graft usually weighs 800-1000g and, thus, is appropriate for recipients weighing 80-100kg. Left lateral split grafts are primarily used for pediatric recipients. A majority of left lateral section grafts are transplanted into children younger than 5 years (30% being used for recipients less than 1-year-old and 50% of the grafts into 1-year-old to 5-year-old recipients).
- The technique: Splitting of the liver can occur in situ or ex vivo. Technique may be dependent on surgeon experience, donor stability, equipment availability at donor or recipient hospitals, and allocation policy regarding sharing of the second graft. In situ division allows for decreased cold ischemic time, easier recognition of anatomic variants that may be prohibitive for splitting, improved hemostasis of the cut surface, and ligation of biliary radicals. Ex vivo division reduces donor OR time and may be performed at surgeon’s own hospital. An on-table (in situ) or back-table (ex vivo) cholangiogram can also be utilized to evaluate the anatomy prior to the split. A few small volume studies suggest ex vivo splits have worse outcomes than in situ splits, but other institutional studies and reviews show no difference. The introduction of normothermic machine perfusion (NMP) and hypothermic oxygenated perfusion (HOPE), potentially increases opportunities for ex vivo splitting.
- Outcomes: Split outcomes are worse than whole grafts when recipients have the following risk factors: donor age >50 years, GRWR <1.0%, retransplantation, and recipient status I to IIa. Therefore, partial grafts can achieve good long-term outcomes similar to full grafts with appropriate donor and recipient selection.
Small for size syndrome (SFSS)
SFSS is a potentially life-threatening condition of progressive liver dysfunction/failure that results from grafts with inadequate functional hepatic mass. It is distinguished from early allograft dysfunction (which is defined as bilirubin > 10 mg/dL, INR > 1.6, or AST/ALT >2000 IU/L in first 7 days). Some loosely define SFSS as persistent cholestasis and coagulopathy with intractable ascites after POD 14. Across the literature, there are a variety of definitions which led to a recent consensus conference (ongoing) to better define SFSS and provide management recommendations.
- Clinical suspicions: SFSS is diagnosed after excluding other causes of graft dysfunction such as technical causes, rejection, and infection. Historically, it was defined as the presence of two of the following on three consecutive days: a) bilirubin > 100umol/L, b) INR >2, c) grade 3 or 4 encephalopathy. Other signs of SFSS include high-volume ascites.
- Pathophysiology: SFSS is caused by high portal vein pressures that lead to microcirculatory disturbances, causing hepatocellular damage and death. Risk factors include donor age, macrosteatosis, graft size, presence of portal hypertension, and hyperdynamic splanchnic circulation. The ILTS-iLDLT-LTSI Consensus Conference recently published recommendations for defining/diagnosing SFSS, strategies to reduce risk of and mitigate impact of SFSS.
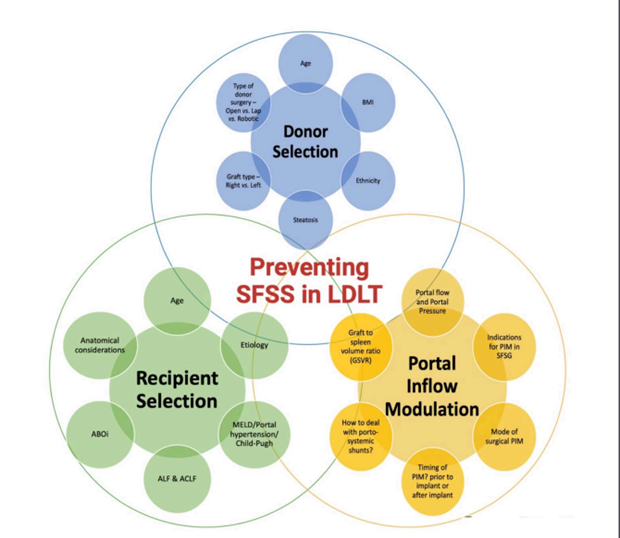
Figure 11: Factors to be considered for preventing SFSS in LDLT.
3. Recommendations for avoiding SFSS (Figure 11):
a. Age: Use grafts with donor age of < 45 years
b. Graft size: Liver grafts which provide GRWR greater than 0.8% and a graft weight to standard liver volume ratio (SLV) greater than 40% are at lower risk for SFSS. As such, the larger right lobe is preferred to the left lobe (especially when there is borderline graft volume).
c. Portal venous pressures: Early post-operative portal pressure > 20 mmHg is associated with rapid graft hypertrophy, SFSS, and poor patient survival. Portal inflow modulation should be considered when intraoperative portal venous pressure to the graft exceeds 15mmHg or portal flow exceeds 250mL/min/100g. Technical options include splenic artery ligation, splenectomy, and creation of portosystemic shunts. Such techniques are associated with lower risk of SFSS and overall improved outcomes.
d. Outflow optimization: Suboptimal venous outflow results in graft congestion and can impair impaired regeneration. This can reduce functional capacity of the graft. This is particularly important in right lobe grafts without the middle hepatic vein. Reconstruction of large segmental veins (that contribute more than 10% outflow to the graft) should be considered.
e. Recipient optimization: The status of the recipient before transplant can influence postoperative development of SFSS. Survival after transplant with a partial graft, but not whole grafts, is lower for Child B and C recipients versus Child A. SFSS also develops more frequently in recipients with cirrhosis versus non-cirrhotics. Ultimately, the severity of the recipient's disease needs to be contemplated when considering partial grafts.
f. Donor factors: Underlying liver disease, such as steatosis, can reduce the effective liver mass. While the exact amount of steatosis that can have a deleterious effect is unknown, in general it is recommended that larger grafts be procured from steatotic livers. The current recommendations suggest use of grafts with macrosteatosis of <20%.
Take home points:
- While living donor liver transplantation offers an alternative source for liver transplant candidates with limited access to deceased donor organs, it does require appropriate donor-recipient pairing and graft selection for success.
- Use of pediatric grafts in adults requires special consideration with selection guided by graft weight, with heightened risks small-for-size-syndrome and hepatic artery thrombosis.
- Split liver transplantation offers a solution to address organ shortages while also providing appropriately sized grafts for pediatric recipients – usually the graft is split into a left lateral section (for pediatric recipient) and an extended right lobe (for an adult recipient).
- Small for size syndrome (SFSS)is a potentially life-threatening condition of progressive liver dysfunction/failure that results from grafts with inadequate functional hepatic mass. Risk factors include donor age, macrosteatosis, graft size, presence of portal hypertension, and hyperdynamic splanchnic circulation.