Bile Duct Neoplasms: Focus on Bile Duct Adenoma and its Evaluation in Intraoperative Consultation
Brief Case Presentation
This is a case of an intraoperative consultation for donor liver evaluation. The donor passed away due to congestive heart failure, with no significant oncological history. During intraoperative organs evaluation, surgeons noticed a 1-cm superficial/subcapsular nodule in the left hepatic lobule that was not previously detected on the preliminary CT assessment.
A wedge biopsy including the nodule was performed and sent for intraoperative consultation/frozen section assessment.
***
Microscopic Findings
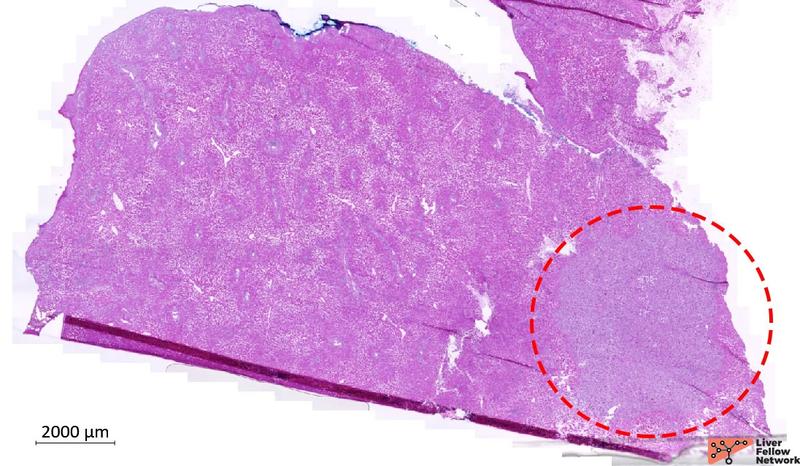
The specimen consists of a tan, firm wedge of liver parenchyma measuring 1.5x1x0.5 cm. On the cut surface, there is a white-grey firm nodule measuring approximately 0.5x0.4x0.2 cm. Low-power analysis confirmed the presence of a 5-mm nodule located in the subcapsular hepatic parenchyma (Figure 1).
Figure 1. Liver wedge biopsy, low-power image of the frozen tissue available for analysis. Red-dotted circle outlines the nodule. [Hematoxylin and eosin stain; frozen section].
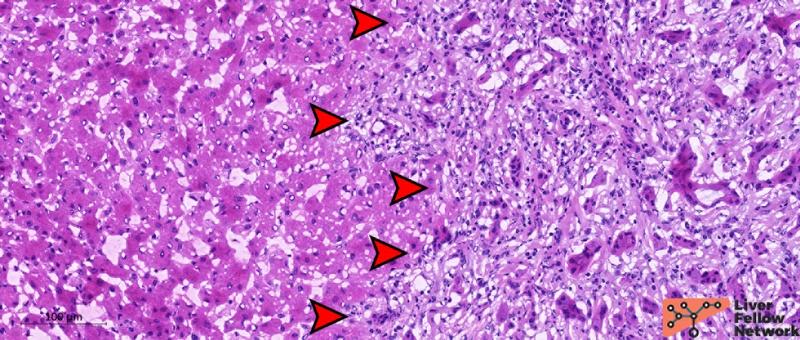
At medium-power, the nodule (Figure 2) is composed of a ductular proliferation embedded in a fibrotic stroma. The nodule has no capsule but is well-circumscribed and distinct from the surrounding liver parenchyma, with no infiltrative or pushing margins.
Figure 2. Medium-power details of the nodule. Red arrows indicate the limit of the nodular area. The nodule is unencapsulated but well-defined and distinct from non-neoplastic surrounding hepatic parenchyma, with no signs of infiltrative margins. [Hematoxylin and eosin stain; frozen section].
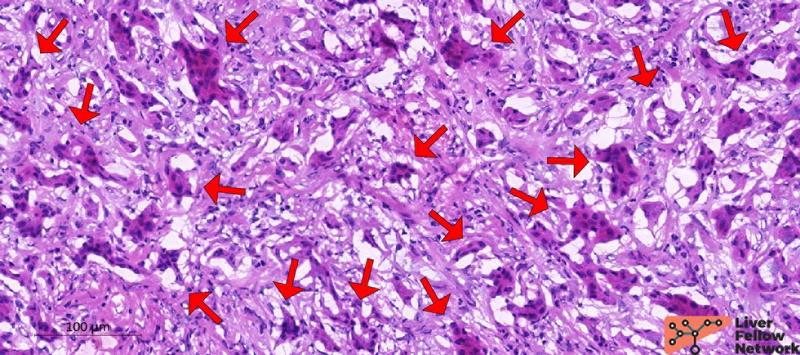
The ductular structures are small and uniform, consisting of bland-appearing biliary-type cuboidal/columnar cells with a low nuclear-cytoplasm ratio, minimal atypia and no evident mitotic activity (Figure 3).
Figure 3. High-power details of the glandular structures. Red arrows indicate relatively normal ductules composed of biliary-type epithelial cells with minimal atypia, no evident mitosis, and a low nuclear-cytoplasm ratio. [Hematoxylin and eosin stain; frozen section].
In summary: This is a single small incidental lesion with subcapsular location, occurring in the liver of a patient with no oncological history, with the histopathological changes described in Figures 1-3. What is the preliminary/intraoperative diagnosis?
***
This is a Bile Duct Adenoma.
***
Bile duct adenoma: definition, etiopathogenetic hypothesis, and WHO classification.
Bile duct adenoma (BDA) is a benign neoplasm originating from biliary epithelial cells, characterized by a proliferation of small, bland, biliary ductules. These can occur at any age, with reported cases in both pediatric and adult populations, and show no sex predilection. BDA is relatively rare, representing approximately 0.5-2% of hepatic primary lesions. However, it is commonly an incidental finding identified during abdominal surgery for other causes (as in this case) or at autopsy, making it challenging to accurately estimate its true incidence. Definitive diagnosis relies on histopathological assessment, as no tumor serological biomarkers or imaging characteristics allow definitive preoperative characterization [1, 2].
The biological nature and neoplastic nature of BDA are still debated. On one hand, some propose that BDA is not a true neoplastic process, but rather either a peribiliary gland hamartoma (due to its similarity with secretory gland cell phenotype) [3] or a regenerative/reparative phenomenon secondary to a specific, self-limited local injury (due to its typical subcapsular/peripheral location where traumatic/ischemic injuries are frequently observed) [4].
On the other hand, BDAs share features that are used to define neoplastic process with putative malignant potential. Some BDAs have shown focal atypical features and reports of progression to cholangiocarcinoma [5, 6] or co-existence with cholangiocarcinoma [7]. Detection of BRAF V600E mutation in selected BDA series (up to 53%), is also suggestive of a clonal/neoplastic nature [8, 9].
As none of these hypotheses have been fully confirmed so far, the most recent version (5th Edition) of the World Health Organization (WHO) classification of digestive system tumors discusses both the reactive and neoplastic etiologies of this entity, pending further longitudinal studies and clinical, morphological, and molecular characterization [10].
Histopathology of BDA: typical features, diagnostic differentials, and potential diagnostic pitfalls in intraoperative consultation.
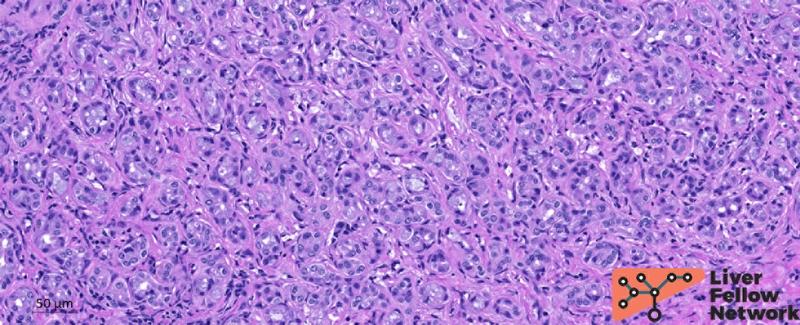
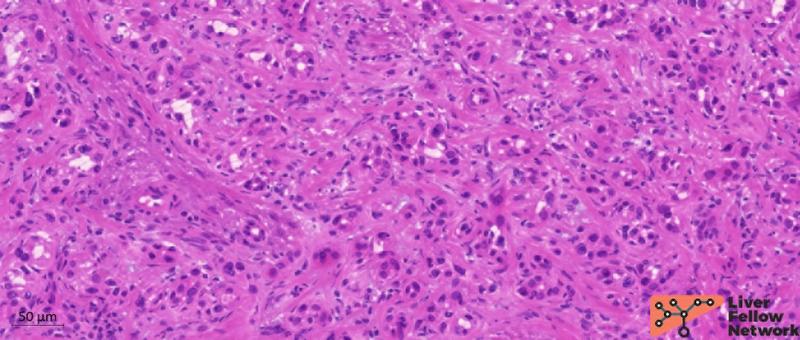
Macroscopically, BDA is usually a small (typically <2 cm) solitary lesion located in the subcapsular/peripheral hepatic parenchyma, presenting as a firm, white-gray, well-circumscribed nodule. Histopathologically, BDA is comprised of an unencapsulated nodule with a well-defined border with a smooth interface with the adjacent parenchyma and no pushing nor infiltrative margins. BDA is composed of densely packed, homogenously shaped, and uniformly spaced tubular ductules with pinpoint or absent lumens (Figure 4). These ductules are typically disordered with no specific pattern of growth and present preserved basement membranes. Branching appearance and intra-luminal mucin is variably present.
Figure 4. Medium-power representation of BDA ductules pattern. Ductules are small and homogeneously distributed with no specific pattern.[Hematoxylin and eosin stain; formalin-fixed paraffin-embedded section].
Entrapped portal tracts within the nodule and a fibrotic stroma with variable chronic inflammation are also common findings (Figure 5).
Figure 5. Common histopathological features of BDA. BDA can frequently present entrapped portal tracts (A; red arrows), chronic inflammatory infiltrates (B; red arrows), and fibrotic stroma (C). [Hematoxylin and eosin stain; formalin-fixed paraffin-embedded sections].
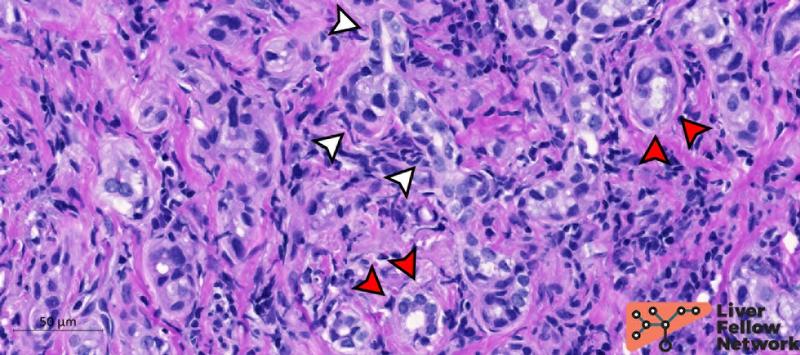
BDA tumor cells are cuboidal to columnar with no atypia or mitosis, typically organized in a single layer (Figure 6).
Figure 6. Details of BDA cells. Ductules composing BDA presents a single layer of cuboidal/columnar cells with no atypia (red arrows) resembling typical biliary epithelial cells. Ductules can occasionally present branching architecture (white arrows). [Hematoxylin and eosin stain; formalin-fixed paraffin-embedded section].
In summary, the main histopathological features of BDA are:
- Peripheral, single, small, unencapsulated nodule with well-defined margins;
- A proliferation of small, relatively uniform ductules in a fibrotic connective tissue;
- Ductules with a single layer of cuboidal/columnar cells with no atypia or mitosis;
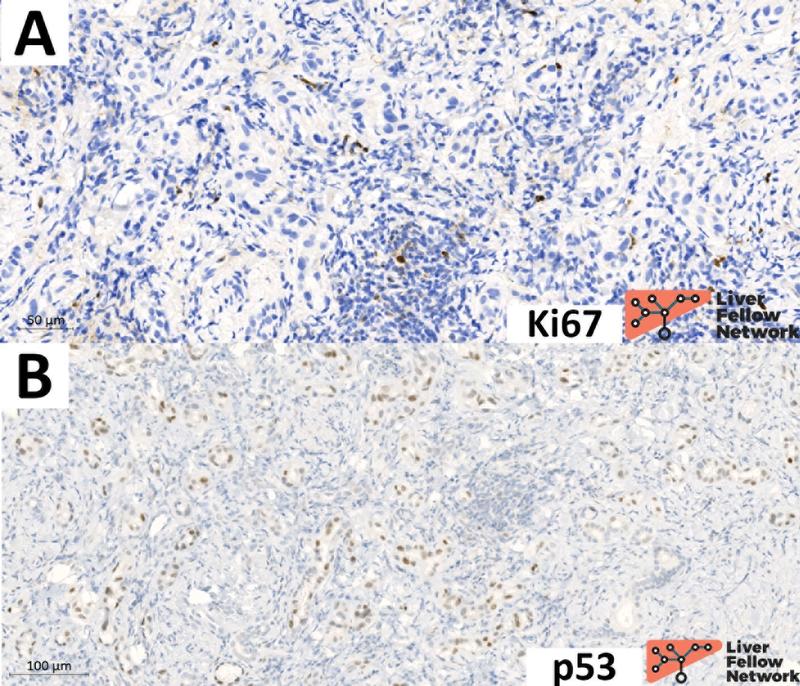
The differential considerations for BDA include other benign biliary proliferations, such as ductular proliferation and bile duct hamartomas (Von Meyenburg Complex). Although other neoplastic differential considerations are pancreatic-biliary tract cancers (i.e., well-differentiated intrahepatic cholangiocarcinoma and hepatic metastasis from pancreatic adenocarcinoma), the lack of cytologic atypia and lack of infiltrative borders typically excludes a malignant diagnosis. On formalin-fixed paraffin-embedded specimens (FFPE), immunohistochemical stains can support the distinction between these entities [11]. Specifically, Ki67 and p53 staining patterns are informative: BDA presents a very low proliferative index and a wild-type p53 pattern of expression (Figure 7), whereas pancreatic-biliary tract cancers typically present high proliferative index and mutated p53 expression (Figure 8) [12].
Figure 7. BDA presents low proliferative index and wild-type p53 pattern of expression. A) In this case of BDA, the proliferative index (assessed via anti-Ki67 immunohistochemical stain) is 0-1% (essentially no Ki67 nuclear expression on BDA tumor cell); inflammatory cells are the only cell population labeled by Ki67 and served as positive internal controls. B) BDA presents a p53 wild-type pattern of expression, characterized by variable intensity of p53 protein expression (from negative to strongly expressed) in BDA tumor cell nuclei.
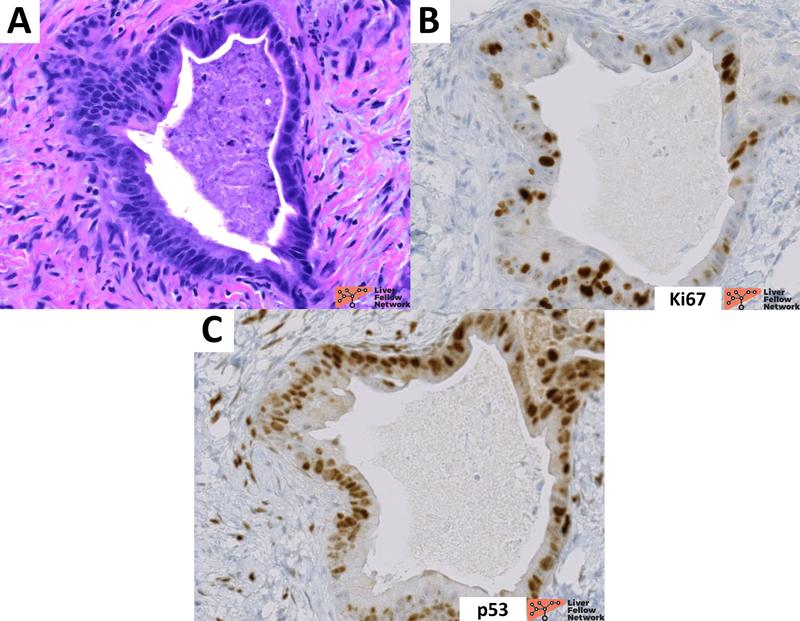
Figure 8. Proliferative index and p53 expression in a cholangiocarcinoma gland. A representative gland of a well-differentiated cholangiocarcinoma case presenting atypical morphology and architecture (A; hematoxylin and eosin). Compared to the profile reported in Figure 7, cholangiocarcinoma presents high proliferative index (B; usually >10% of tumor cell nuclei are positive) and mutated pattern of p53 (C; strong and diffuse protein expression in almost all tumor cell nuclei).
In addition, p16 can also be successfully used, but with some limitations: p16 is positive in BDA but negative in some intrahepatic cholangiocarcinoma. Therefore, it is helpful to exclude BDA when negative [13]. Of note, keratin stains are of limited utility due to the overlapping profiles between BDA and pancreato-biliary tract cancers.
In frozen sections, the differential between BDA and pancreato-biliary tract cancer is challenging, because the freezing process introduces tissue artifacts that may mimic architectural and cellular atypia. In this context, certain features can be especially useful, including:
- clinical history (incidental lesion), tumor size (<2 cm) and location (single, peripheral nodule);
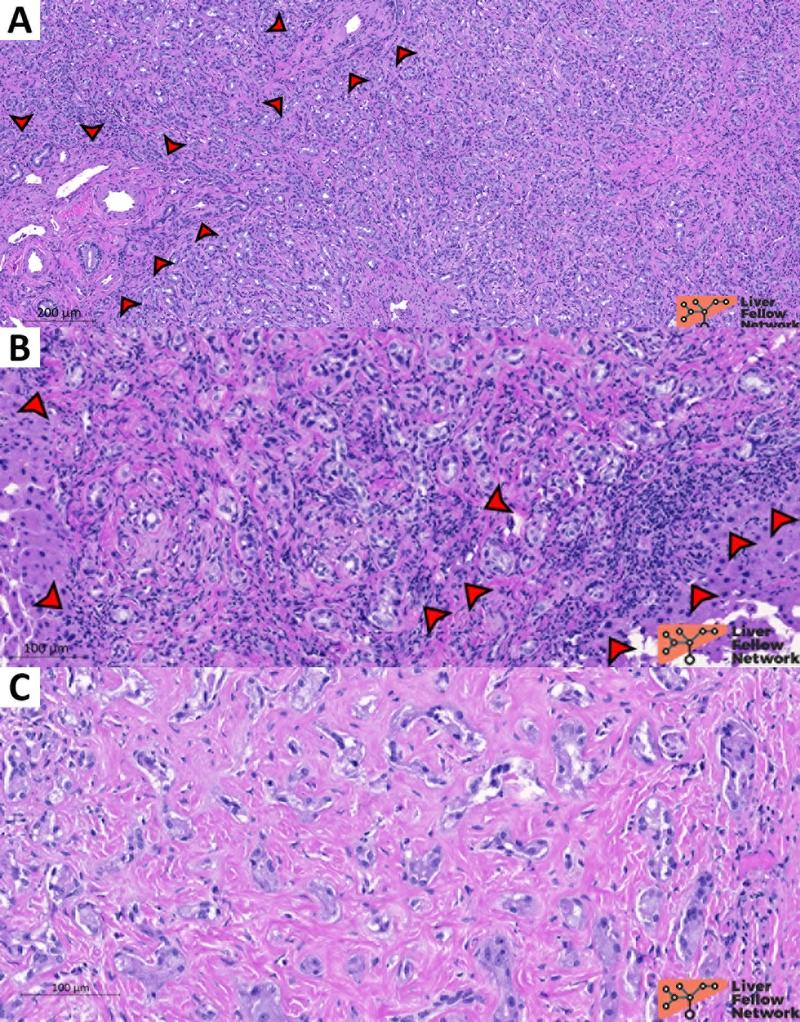
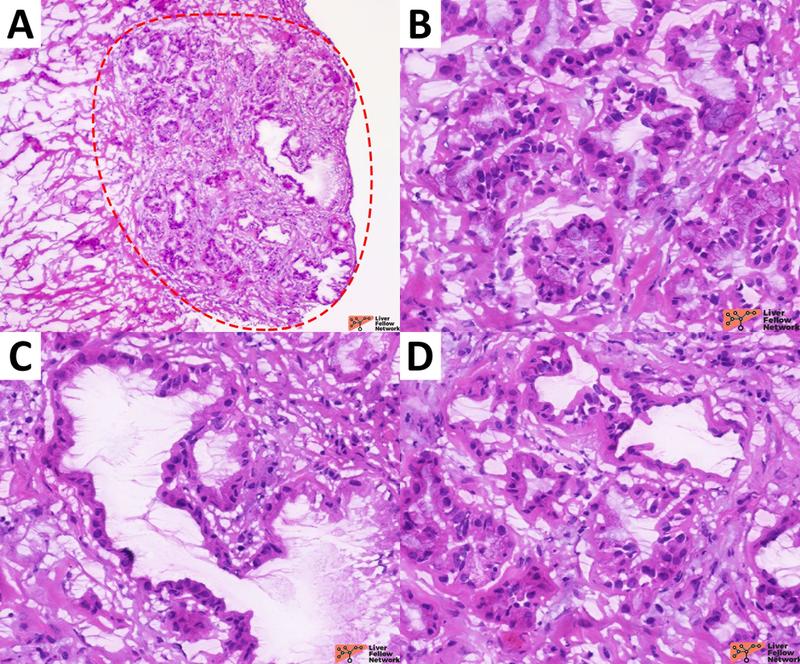
- cytological and architectural patterns: BDA presents homogeneously distributed small rounded ductules without invasive features (Figure 9). In contrast, pancreato-biliary tract cancer often exhibits infiltrative margins and heterogeneous, more atypical ductular structures (Figure 10);
- inclusion of portal tracts within the nodule, despite its small size.
Given the complexity of distinguishing these lesions and related clinical implications involved, second opinion while performing intraoperative consultation on a nodule with ductular formations is generally recommended.
Figure 9. BDA presents small ductules on frozen section. In this additional case of BDA, ductules are small and homogenously distributed. No major atypia is observed despite the frozen procedure. [Hematoxylin and eosin stain; frozen section].
Figure 10. Representative sections of an intraoperative consultation on a cholangiocarcinoma case. In this case, frozen sections were performed to evaluate the distal margin of a cholangiocarcinoma resection. In addition to the different clinical setting, cholangiocarcinoma present infiltrative margins (A; cholangiocarcinoma is highlighted by the red-dotted circles) and highly atypical neoplastic glands with heterogenous shape and spatial distribution (B, C, D). [Hematoxylin and eosin stain; frozen sections].
Conclusion
In summary, BDA is a rare benign epithelial neoplasm of the liver. It manifests as a solitaryperipheral small nodule composed of bland-looking ductules with enclosed portal tracts and varying amounts of fibrotic stroma and chronic inflammation. The distinction between BDA and pancreatic-biliary tract cancers is challenging on both FFPE samples and frozen section, but can benefit from immunohistochemical stains and second opinion consultation, respectively.
***
References
1. Allaire GS, Rabin L, Ishak KG, Sesterhenn IA. Bile duct adenoma. A study of 152 cases. Am J Surg Pathol. 1988;12(9):708-15.
2. Sarcognato S, Sacchi D, Fassan M, Fabris L, Cadamuro M, Zanus G, et al. Benign biliary neoplasms and biliary tumor precursors. Pathologica. 2021;113(3):147-57.
3. Bhathal PS, Hughes NR, Goodman ZD. The so-called bile duct adenoma is a peribiliary gland hamartoma. Am J Surg Pathol. 1996;20(7):858-64.
4. Ferrell L KS, Terracciano L, Wee A. . Tumours and Tumour-Like Lesions of the Liver. In: MacSween’s Pathology of the Liver. 7th ed Elsevier 2018;780-879. 2018.
5. Hasebe T, Sakamoto M, Mukai K, Kawano N, Konishi M, Ryu M, et al. Cholangiocarcinoma arising in bile duct adenoma with focal area of bile duct hamartoma. Virchows Arch. 1995;426(2):209-13.
6. Pinho AC, Melo RB, Oliveira M, Almeida M, Lopes J, Graca L, et al. Adenoma-carcinoma sequence in intrahepatic cholangiocarcinoma. Int J Surg Case Rep. 2012;3(4):131-3.
7. Sasaki M, Sato Y, Nakanuma Y. Bile duct adenoma may be a precursor lesion of small duct type intrahepatic cholangiocarcinoma. Histopathology. 2021;78(2):310-20.
8. Pujals A, Amaddeo G, Castain C, Bioulac-Sage P, Compagnon P, Zucman-Rossi J, et al. BRAF V600E mutations in bile duct adenomas. Hepatology. 2015;61(1):403-5.
9. Pujals A, Bioulac-Sage P, Castain C, Charpy C, Zafrani ES, Calderaro J. BRAF V600E mutational status in bile duct adenomas and hamartomas. Histopathology. 2015;67(4):562-7.
10. WHO Classification of Tumours Editorial Board. Digestive system tumours. Fifth Edition. Lyon (France): International Agency for Research on Cancer; 2019
11. Hornick JL, Lauwers GY, Odze RD. Immunohistochemistry can help distinguish metastatic pancreatic adenocarcinomas from bile duct adenomas and hamartomas of the liver. Am J Surg Pathol. 2005;29(3):381-9.
12. Tsokos CG, Krings G, Yilmaz F, Ferrell LD, Gill RM. Proliferative index facilitates distinction between benign biliary lesions and intrahepatic cholangiocarcinoma. Hum Pathol. 2016;57:61-7.
13. Sasaki M, Matsubara T, Kakuda Y, Sato Y, Nakanuma Y. Immunostaining for polycomb group protein EZH2 and senescent marker p16INK4a may be useful to differentiate cholangiolocellular carcinoma from ductular reaction and bile duct adenoma. Am J Surg Pathol. 2014;38(3):364-9.
***
Written by Alessandro Gambella, MD; PhD candidate - Division of Liver and Transplant Pathology, University of Pittsburgh – Research Histology Services, Thomas E. Starzl Transplantation Institute