Why do we use albumin in patients with cirrhosis?
This one is easy peasy. Liver disease impairs albumin, so let’s give albumin replacement, right? Not so fast. There is A LOT more to albumin than meets the eye. Let's dive in.
What is albumin?
Albumin is the predominant circulating protein in plasma. (Guyton and Hall Textbook of Medical Physiology, 13th ed. Chapter 16.). Some important characteristics:
- The main determinant of intravascular oncotic pressure
- Produced by the liver
- Its half-life is approximately 17-21 days
Aside from its role in maintaining oncotic pressure, albumin has many other important roles:
- Helps permit solubilization and transport of various molecules
- Calcium, magnesium, copper, zinc
- Drugs (warfarin, NSAIDs)
- Long chain fatty acids
- Toxic metabolites (unconjugated bilirubin)
- Inflammatory mediators (e.g., in sepsis)
- Antioxidant capacity
- Acts as a free radical scavenger for reactive oxygen species
- Neutralizes free copper, iron
- Immunomodulation
- Systemic inflammatory responses can be triggered by immune recognition of pathogen-associated molecular patterns (PAMPs are unique microbial-specific antigens such as lipopolysaccharide or peptidoglycans) and damage-associated molecular patterns (DAMPs, endogenous substances such as mitochondrial DNA released by dying cells). Albumin down-regulates the inflammatory response by binding to these molecules, as shown in studies with lipopolysaccharide and prostaglandin E2
- Increases intracellular glutathione (an essential component of normal cellular and humoral immune responses) and regulates activation of the NF-𝝟β pathway (an important pro-inflammatory signaling pathway)
When did we start using it in cirrhosis?
Albumin has been used in cirrhotics basically since the beginning of time...or 1946.
In the early 1940s = human serum albumin used in sailors suffering severe burns in the Pearl Harbor attack during World War II
1946 to 1949 = investigators study IV albumin as a treatment for ascites.
The theory here was that hypoalbuminemia was the primary event in the formation of ascites (low oncotic pressure = capillary leak = ascites).
In these studies, IV albumin increased serum albumin levels and even induced diuresis (evidenced by increased urine volumes and improvements in peripheral edema), but there was no consistent effect on ascites.
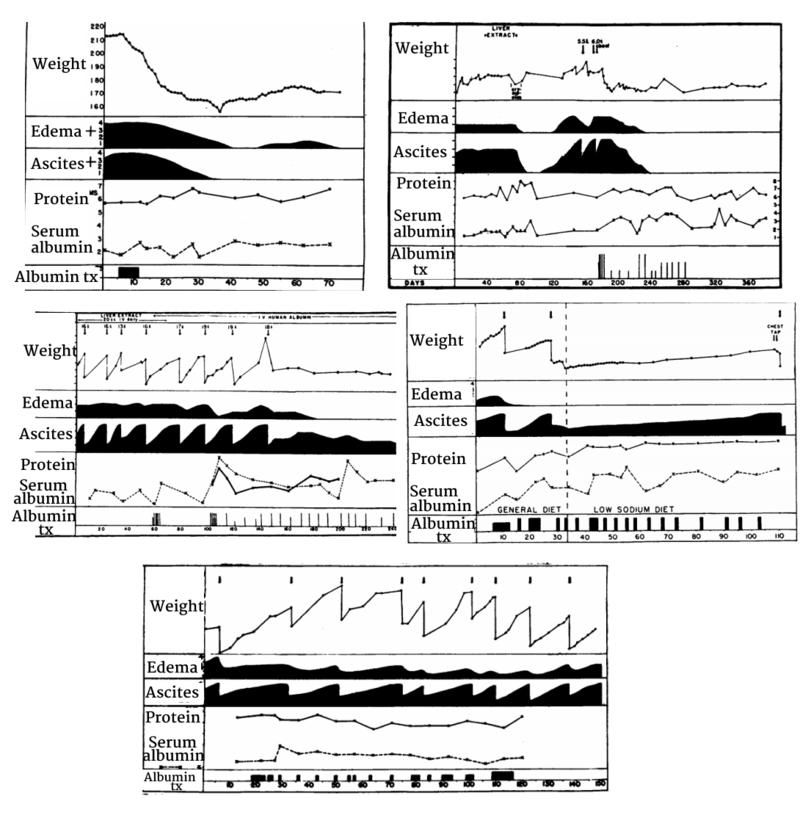
The above figure is taken from Faloon et al. (1949) - showing representative examples of 5 sample patients, in whom some had resolution of ascites while others had recurrent or persistent ascites despite often seeing improvement in edema or increases in serum albumin.
So why might albumin, on its own, not help?
- It’s not just hypoalbuminemia and low oncotic pressure that cause ascites
- Decreased effective arterial blood volume (EABV) due to massive splanchnic pooling of blood is crucial
- ↓ EABV = ↑ RAAS axis and sympathetic nervous system = sodium/water retention = ascites
- So albumin may expand plasma volume temporarily, but it doesn’t affect the extensive splanchnic vasodilation and thus EABV still remains low, RAAS activity remains high, and ascites still forms in many patients
[ Re-read my post on ascites and diuretics for more on this! ]
So what are the indications for the use of albumin in cirrhosis now?
We’ll focused on the 3 most well-studied indications:
- Prevention of post-paracentesis circulatory dysfunction (PPCD)
- Prevention of HRS in patients with SBP
- Differentiation and treatment of HRS
I’ll also touch on some other questions that I personally had as I was writing this article:
- Should albumin be used in addition to antibiotics in all infections (not just SBP)?
- Should we be using albumin in any hypotensive cirrhotic patient?
Indication #1: Prevention of post-paracentesis circulatory dysfunction
After an LVP (typically with ≥5L removed), the reduction in intra-thoracic pressure increases venous return to the heart = increase in cardiac index
The heart: “Yippee, let’s pump out all this blood!”
[Cardiac index increases]
Baroreceptors: “Finally, you filled the tank! Now we can all relax a bit.”
[SVR drops]
The arteries: “Wait, wait! We’re still effectively depleted!”
[Effective arterial blood volume (EABV) drops]
Baroreceptors: “Oopsies.”
The kidneys: “Sigh. Here we go again…”
[ RAAS and SNS increase dramatically in response to the decrease in EABV ]
Post-paracentesis circulatory dysfunction (PPCD) refers to the cardiovascular and hemodynamic changes that occur as a consequence of large-volume paracentesis (LVP). It is defined by an increase in plasma renin activity > 50% baseline.
The deleterious effects can include significant hyponatremia, renal dysfunction, and precipitation of HRS.
In 1988, Gines et al. first described the favorable effects of albumin in patients undergoing paracentesis, showing ↓↓ renal dysfunction and hyponatremia.
Other colloids (dextran-70, polygeline) have been tried with less success compared to albumin.
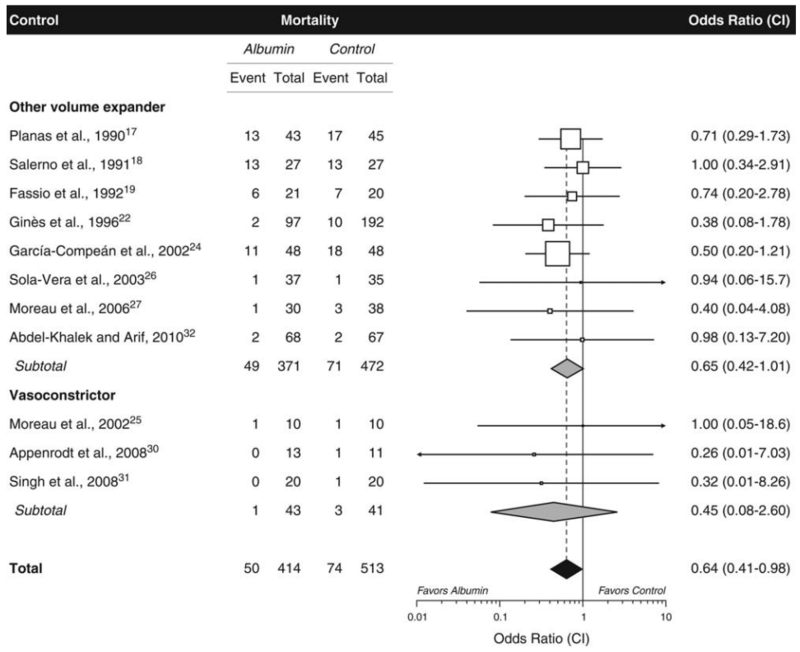
In a systematic review/meta-analysis by Bernardi et al., compared with alternative treatments (other volume-expanding agents or vasoconstrictors), albumin was associated with significant reductions in PPCD, hyponatremia, and mortality.
Below is a figure from the Bernardi paper comparing risk of mortality with albumin vs. other treatments
Here again, we are seeing data suggesting that the benefits of albumin extend beyond its ability to increase plasma volume.
Current recommendations:
In patients undergoing LVP (5L or greater), administer albumin (6-8g/L ascites removed of 20 or 25% albumin solution) in order to reduce the risk of PPCD and its associated complications.
Indication #2: Prevention of HRS in patients with SBP
In the early 1990s, it was shown that 1/3rd of inpatients with SBP developed renal impairment. In many, the renal injury was sustained or progressive, and it was the strongest independent predictor of in-hospital mortality.
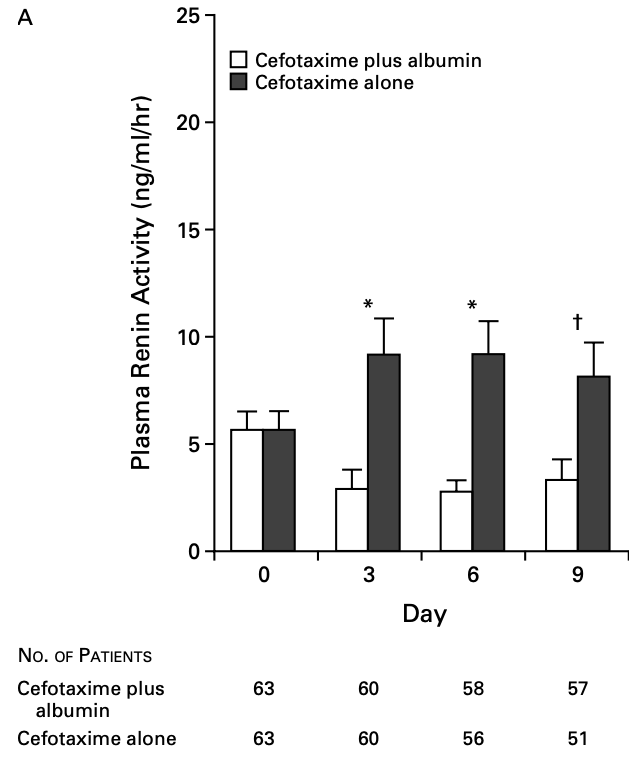
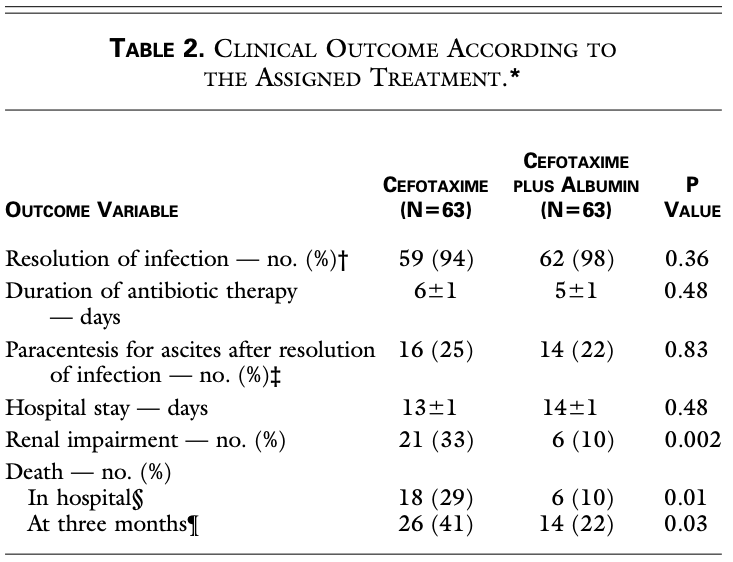
In 1999, Sort et al. published a landmark RCT of 199 inpatients with cirrhosis and SBP, comparing antibiotics alone to antibiotics and 20% albumin (1.5g/kg on day 1 and 1g/kg on day 3). In the antibiotics+albumin group, they found:
- Similar rate of resolution of SBP (94 vs 98%)
- Significantly ↓↓ plasma renin levels
- Significantly ↓↓ renal impairment (10% vs 33%)
- Significantly ↓↓ in-hospital (10% vs 29%) and 3-month mortality (22% vs 41%)
On the basis of this study, albumin became standard practice in preventing HRS and reducing mortality in all patients with SBP.
However, there do remain some open questions regarding the study, including whether ALL patients with SBP will benefit from albumin.
First, let’s discuss some interesting points related to the study design:
- The dose of albumin in this study was arbitrary
- Let me repeat...the dose of albumin in this study was arbitrary!
- There was no volume strategy used in the control group (i.e. crystalloids, etc) as a comparison to albumin
- No information was provided regarding the management of renal impairment in the control group. Were the patients treated with IV fluids? Was vasoconstrictive therapy offered? What was their “standard of care”?
- Patients with evidence of “dehydration” were excluded -- the authors mention that this was determined by history of “diarrhea” or “intense response to diuretics in the week prior to SBP.” It is not clear how they defined an intense diuretic response. In some patients (15 total), CVP was measured, and patients with CVP < 4mmHg were excluded.
Did albumin actually provide additional benefits over “standard of care” or crystalloid therapy? We can't really answer this.
Also: if you were less sick at baseline, you had an exceedingly ↓↓ incidence of renal impairment!
- 34 patients in the study had baseline bilirubin < 4 mg/dL and creatinine < 1mg/dL (less sick)
- Risk of renal impairment in these patients: 0% in albumin group, 7% in controls
Perhaps there is a “low-risk” group that may not require albumin?
There are subsequent small studies that have suggested the same:
In 2007, Sigal et al. published on a small series (28 patients with 38 SBP episodes) who were given albumin only when baseline bilirubin was > 1mg/dL and creatinine > 1.0mg/dL. In the group that did not meet these criteria (i.e. bilirubin < 1, Cr < 1) where albumin was not given, there were no episodes of renal impairment or death.
Additional, larger studies are likely necessary before this can be universally adopted.
Current recommendations:
In patients receiving treatment for spontaneous bacterial peritonitis, 20-25% albumin administration at a schedule of 1.5g/kg on day 1 and 1g/kg on day 3 is recommended to reduce the risk of HRS and mortality.
Based on this, why don’t we administer albumin for all non-SBP infections in cirrhotics?
This is a valid question. If the inflammatory state of SBP can precipitate HRS, it might be that albumin helps in all infectious situations. Thankfully, there are three RCTs looking at this.
Two earlier RCTs, one by Guevara et al. in 2012, and another by Thevenot et al. in 2015 both studied hospitalized patients with cirrhosis and non-SBP infections (PNA, UTI, SSTIs, etc). Patients were randomized to antibiotics +/- albumin (given in the same day 1 and day 3 protocol as in the Sort SBP study).
In the Guevara study, the median MELD was 16-17 and they found:
- Albumin = ↑↑ renal function (serum creatinine and GFR)
- No effect on survival or mortality (in intention-to-treat analysis)
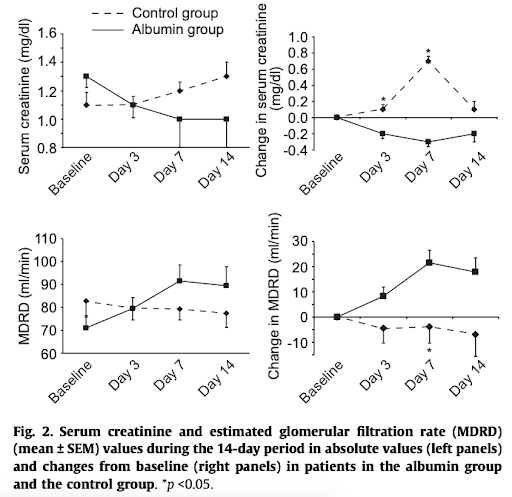
In the Thevenot trial, all patients had a Child-Pugh score > 8 and sepsis (defined as 2+ SIRS criteria with a documented infection). They found:
Finding #1: no difference in rate of renal failure (13% vs 14%) at 3-month
However, longer median time to onset of renal failure with albumin (29 vs 12 days)
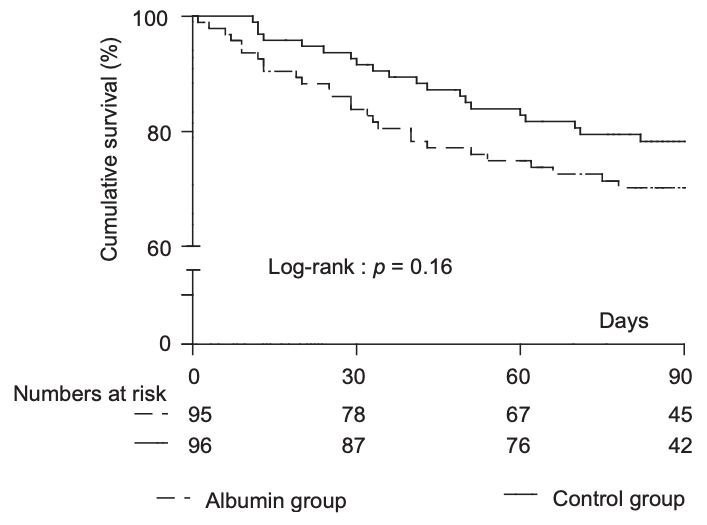
Finding #2: no difference in overall 3-month survival (see below graph)
Finding #3: ↑↑ risk of pulmonary edema (8.3%) in the albumin group. The rate of pulmonary edema in the control group was not reported.
Finally, a very recent trial by Fernandez et al. in 2020 of 118 cirrhotics with non-SBP infections aimed to look at a slightly sicker population by:
- Identifying predictors of mortality:
- Serum Cr > 1.2 mg/dL
- Na ≤ 130 mEq/L
- Bilirubin ≥ 4 mg/dL)
- Requiring that patients had at least 1 of these predictors
- Also required that lower-risk infections (e.g., UTI) had a 2nd of the above predictors + SIRS criteria + CRP ≥ 1 mg/dL
Their findings:
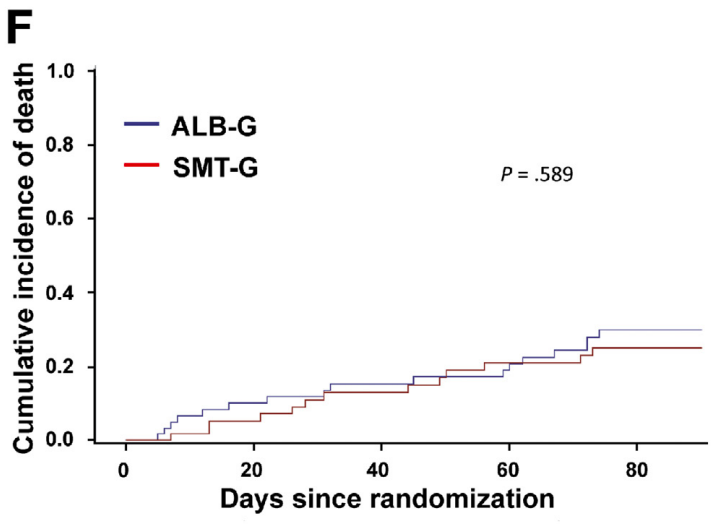
Finding #1: No difference in mortality (see below) or liver transplantation
Finding #2: Higher rate of resolution of acute-on-chronic liver failure with albumin (82.3 vs 33.3%)
Finding #3: Decreased rate of nosocomial infections with albumin (9.8 vs 24.6%)
Finding #4: More significant decreases in CRP, IL-6, and WBC in the albumin group
Takeaways:
There is no great evidence that albumin adds much in the treatment of non-SBP infections in cirrhosis. However, more recent data suggest possible benefits outside of mortality reduction (reduction in nosocomial infections and ACLF) that need to be explored further.
Indication 3: Treatment of HRS
Hepatorenal syndrome (HRS) is a serious complication of progressive liver disease involving a complex interplay between circulatory dysfunction, renal physiology, and systemic inflammation.
Good evidence exists that the addition of albumin to a vasoconstrictor increases response rates in the treatment of HRS.
The most commonly-cited study to this effect was a small, non-randomized study by Ortega et al. of 21 patients with HRS treated with terlipressin. 13 of the 21 patients received concomitant albumin (20% solution at dose of 1g/kg of body weight per day for first 2 days and then 20-40g/day thereafter). Most germane to this discussion were the following findings:
Of the 12/21 (57%) who showed complete response (Cr < 1.5), treatment with albumin was the only predictive factor of this outcome (77% of terlipressin+albumin vs 25% of terlipressin alone showed complete response)
Takeaways:
Co-administration of vasoactive therapy with albumin, at a dose of 20-40g/day of 20-25% albumin, appears to be beneficial in the treatment of HRS.
I’ve always wondered, should I be preferentially using albumin over crystalloids in my cirrhotic patients who are hypotensive or in shock (e.g., severe sepsis)?
This is a complicated question with no great answer.
The colloid-crystalloid debate in critical care in general has raged on for years. The most recent consensus, according to the Surviving Sepsis Campaign recommendations, are:
- Crystalloids are the fluid of choice for initial resuscitation and subsequent intravascular volume replacement in sepsis and septic shock (strong recommendation, moderate quality of evidence)
- Use albumin in addition to crystalloids when patients require substantial amounts of crystalloids (weak recommendation, low quality of evidence)
I would suggest reading the following for some additional background information on the above recommendations.
- 1998 Cochrane Review -- review of 30 RCTs showing 6% increase in mortality in critically-ill patients receiving albumin compared to crystalloids. This led to a move away from using albumin in the ICU
- Saline versus Albumin Fluid Evaluation (SAFE) trial -- 2004 NEJM RCT of 4% albumin vs normal saline in 7,000 critically-ill patients, showing no mortality difference. However, interestingly, in a pre-defined subgroup with severe sepsis, there was a mortality benefit.
- ALBIOS trial -- ICU-based RCT with ~1800 patients comparing 20% albumin + NS vs. NS alone. Albumin was only given to a target of ≥3g/dL. This also showed no significant differences in survival at 1 or 3 months, however in the subgroup with severe septic shock, there was a suggestion of a survival benefit.
*Note that the above two studies (SAFE and ALBIOS) excluded patients with cirrhosis*
At this point, there are no studies looking at albumin vs. crystalloid specifically in cirrhotics with severe sepsis, septic shock, etc.
Takeaways:
We’re in a so-called “evidence-free zone” here so there’s no straight answer. It seems that albumin may actually have some benefit in very sick patients in general. But for now we should probably follow critical care guidelines and use normal saline as our first choice, with albumin reserved in those who do not respond.
That's all. Thanks for reading! Hope you learned something.