Why does treatment of hepatocellular carcinoma require a multidisciplinary approach?
The therapeutic approach to any malignancy is a challenging endeavor due to complex pathophysiology and a panoply of treatment options in modern medicine. Cancer is, as Dr. Siddartha Mukherjee puts it, “the emperor of all maladies, the king of terrors.” This is particularly apt for hepatocellular carcinoma (HCC), which is the 3rd cause of cancer-related mortality worldwide and has a dismal 5-year survival below 20%. The low survival rate is primarily driven by diagnosis at late-stage where the median survival is 1-3 years compared to 10 years in early-stage HCC. HCC is one of the fastest growing cancers in the United States and one of the few with a 5-year survival rate of less than 20% and an incidence-to-mortality ratio near 1. The coexisting morbidity of cirrhosis and hepatic dysfunction leads to poor prognosis.
To better understand the multiple therapies available for HCC, we need to understand an effective staging system that funnels therapeutic options by tumor characteristics and prognosis.
How do we stage HCC?
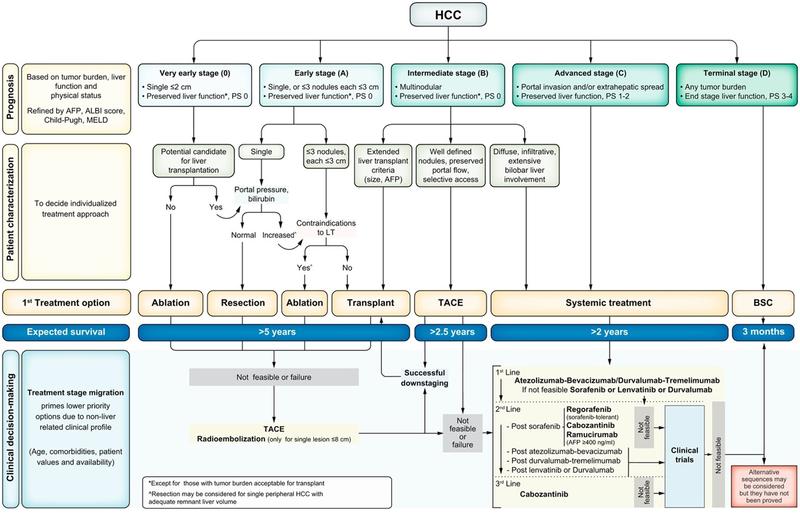
The most widely used staging system for HCC is the Barcelona Liver Clinic Cancer (BCLC). BCLC stratifies HCC by liver dysfunction and Eastern Cooperative Oncology Group performance status (ECOG PS), ultimately guiding best available therapeutic options with prognosis as a corollary. The BCLC was proposed in 1999 at a time when other staging systems (like the Okuda classification) were built on prior studies when the diagnosis was generally made at a more advanced stage with poor life expectancy. The BCLC incorporated the knowledge of earlier diagnosis by advocating for potentially curative treatment options such as surgical resection, liver transplantation (LT) and adjuvant local therapies for early/very early stage patients, while systemic therapies were reserved for more advanced stages (Figure 1).
Figure 1: Updated Barcelona Clinic Liver Cancer (BCLC) staging and treatment strategy in 2022
A 2005 University of Michigan cohort study of 239 patients with HCC and cirrhosis compared seven prognostic staging systems: 1) Okuda, 2) tumor node metastasis (TNM), 3) Cancer of Liver Italian Program (CLIP), 4) BCLC, 5) Chinese university prognostic index (CUPI), 6) Japanese integrated system (JIS), and 7) Groupe d’Etude de Traitement du Carcinoma Hepatocellulaire (GRETCH), in predicting survival. Independent predictors of survival were performance status (p < 0.0001), MELD score >10 (p = 0.001), portal vein thrombosis (p = 0.0001), and tumor diameter greater than 4 cm (p = 0.001). The BCLC had the highest independent power of survival when compared to other six systems (Figure 2).

Figure 2: Probability of survival according to individual HCC staging system. BCLC is “B”
It is important to note that while TNM staging can be used for HCC (like any other malignancy), it does not account for intrinsic hepatic dysfunction (cirrhosis or other liver disease) which drives mortality in HCC. The Child-Pugh classification is used as an adjunct staging classification in HCC, but only accounts for hepatic dysfunction and not for malignancy. An optimal staging system needs to incorporate the 1) severity of underlying disease, and 2) size and degree of spread of tumor. Thus, the BCLC staging system is effective and has demonstratable prognostic value. Unsurprisingly, it is strongly recommended by the AASLD guidelines.
How is HCC managed?
Now that we have discussed staging HCC, we can delve into the various treatment modalities.
Surgical resection:
Surgical techniques have developed rapidly over the last century in improving outcomes in patients undergoing hepatobiliary surgery. Historically, the inception of liver surgery lagged other organs due to organ size, vascularity, intrinsic dysfunction in patients with cirrhosis, and clotting problems due to the liver’s complex role in the fibrinolytic cascade. Earliest partial hepatectomies were performed in the 1880-1890s for metastatic cancer, hemangioma, and adenomas.
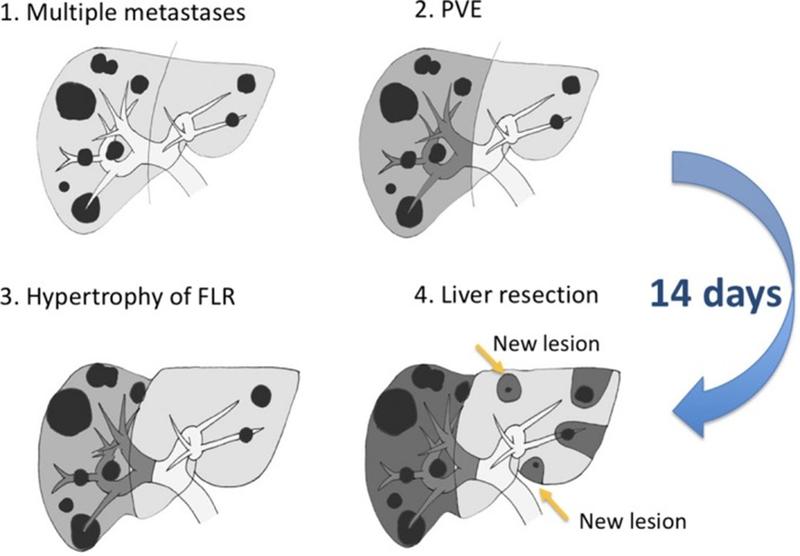
There has been an accelerated improvement in surgical techniques with the addition of intraoperative ultrasound, which has improved the mortality and safety of the procedure by charting vascular anatomy in real time. Preoperative portal vein embolization (PVE) is an adjunct technique that has increased safety and extended indications for major hepatic resections. The concept of preoperative PVE is to induce atrophy of resectable segments and to encourage hypertrophy of remaining segments. The surgeon can evaluate for appropriate healthy tissue post-resection by calculating the anticipated “future liver remnant” or FLR (Figure 3).
Figure 3: Portal vein embolization (PVE) in a patient with multiple metastasis and R lobectomy with subsequent hypertrophy of future liver remnant (FLR)
Surgery is the recommended treatment in patients with localized HCC without cirrhosis or Child Pugh A cirrhosis (no evidence of portal hypertension). However, there is a high rate of recurrence after resection (over 50% over time according to a multicenter study of 404 patients from the International Cooperative Study Group on HCC). This is the reason pre-surgical planning has strict considerations for tumor to be confined to one lobe of the liver and ideally the uninvolved liver tissue to be non-cirrhotic. FLR is defined as 30-40% in patients with cirrhosis, cholestasis, or steatosis, while it is 20-30% in patients with absence of intrinsic liver disease.
Surgical resection requires multidisciplinary care (MDC) discussion as there is high risk of recurrence post-resection which could merit adjuvant therapy including systemic options. Post-operative surveillance is crucial and is recommended with contrast-enhanced multiphase CT or MRI every 3 to 6 months (strong recommendation, AASLD).
Liver-transplantation:
No history of liver transplantation (LT) can be complete without mentioning Dr. Thomas Starzl’s mammoth contributions to the field. Dr. Starzl performed the world’s first liver transplant in 1963 in a 3-year-old patient with advanced biliary cirrhosis secondary to congenital atresia of bile ducts. Patient unfortunately died on the operating table from hemorrhage due to severe coagulopathy and high-pressure venous collaterals. Lessons learnt from this experience advanced the field of LT by Dr. Starzl’s pioneering of cyclosporine in the 1970s and tacrolimus in the 1990s. Success in immunosuppression also advanced care for transplantation of other organs.
The survival of patients undergoing LT for primary and metastatic hepatic malignancies was low in the 1980s. In a retrospective study of 637 patients, the overall recurrence rate was 40% with 81% of deaths from recurrence occurring within 2 years post-LT. UCLA was one of the earliest institutions leading LT. A 1986 study from Busuttil et al documented three LTs for primary hepatic malignancies out of a total 50 LTs. Survival was 33% in LTs for primary hepatic malignancy. Poor early outcomes in LT for HCC led to the US Department of Health and Human Services identifying HCC as a contraindication for LT in 1989.
Poor LT outcomes for patients with HCC were primarily driven by a lack of standardized eligibility criteria on tumor size and number. A 1993 study from the Paul Brousse group in France compared three-year survival with and without recurrence in patients with cirrhosis and HCC (60 patients undergoing resection and 60 patients undergoing LT). Although overall survival rate was similar (50% vs 47% respectively), recurrence-free survival was better with LT over resection (46% vs 27%, p < 0.05). Simultaneously, the Milan multicenter experience was published in 1994 on LT for patients with HCV cirrhosis, HBV cirrhosis, primarily biliary tumors, and HCC.
Dr. Vincenzo Mazzafero et al built up on the Milan mulicenter experience from these studies in 1996 to publish the seminal “Milan criteria” study in the New England Journal of Medicine. This study became a major inflection point in the field of LT for HCC. Patients were carefully selected with eligibility for LT with one tumor 5 cm or less or no more than three nodules, each 3 cm or less, in absence of vascular invasion or extrahepatic metastases. After 4 years, the actuarial survival rate was 75% and recurrence-free survival was 83%. Patients who met predetermined criteria had a an even higher survival rate, overall at 85% and recurrence-free at 92%. This criterion, widely known as the Milan criteria, allowed Medicare to justify paying for LT in patients with HCC in 2001.
Although Milan criteria has become the standard of care and accepted by the United Network for Organ Sharing (UNOS), there are several other criteria that have been proposed to expand eligibility for LT with acceptable post-transplant survival outcomes. These include 1) University of California, San Francisco (UCSF) criteria, 2)total tumor volume (TTV) criteria, 3) “up-to-seven” criteria, 4) Extended Tokyo Criteria and the 5) Kyoto Criteria.
Given scarcity of donor livers, living donor liver transplantation (LDLT) has been explored as an option for patients with HCC. A recent study used the UNOS national database from 1998 to 2018 found 20,161 patients underwent LT with HCC with only 3.6% patients receiving LDLT. The 5-year post-LT survival (LDLT 77% vs DDLT 75%) and HCC recurrence (11% vs 13%) were essentially similar. It is expected that LDLT will grow as an option for patients with HCC due to ongoing scarcity of donor livers.
Given that many patients with HCC often do not have high MELD scores and would be unlikely to receive a LT based on MELD alone, patients meeting Milan criteria receive MELD exception, receiving a score 3 points lower than the median MELD at transplant (MMaT-3) for the area of distribution, defined as a concentric circle around the donor hospital. However, there is a mandatory 6-month waiting period prior to being conferred MELD exception. This opens the door for neoadjuvant therapies (including ablation and chemoembolization) to “downstage” a patient not previously meeting Milan criteria, to meet Milan criteria, prior to LT.
Therefore, LT is the treatment of choice for HCC especially in early stages with significant portal hypertension, as the transplanted liver will replace the recipient liver with poor hepatic function and provide the largest resection margin. The primary limitation of expanding eligibility criteria despite acceptable long-term outcomes is the limited availability of donor livers. MDC plays an important role as LT is a massive undertaking requiring tremendous societal and healthcare resources, given donors livers are a limited resource. Thousands of patients die on the waiting list for a liver transplant; this does not account for the patients who were either removed from the waiting list for being “too sick” to receive a transplant or unable to meet criteria to be on the waiting list.
Local ablation:
Transhepatic localized tissue destruction (or ablation) is a locoregional therapy that can be used as a thermal modality. The principle is inducing selective tumor necrosis by a provoking agent. Ablation is offered to patients either with very-early-stage HCC tumors <2 cm (per BCLC staging) or in patients with small tumors where resection or LT is not possible.
Thermal ablation uses either 1) radiofrequency ablation (RFA), or 2) Microwave ablation (MWA), or 3) Cryoablation to induce thermal destruction of tumor. RFA generates heat by directly passing electric current through the tumor and surrounding tissue (Figure 4). Results of two randomized controlled trials show that RFA and surgical resection had no significant differences in overall or recurrence-free survival, with RFA favored with lowered rates of complications.
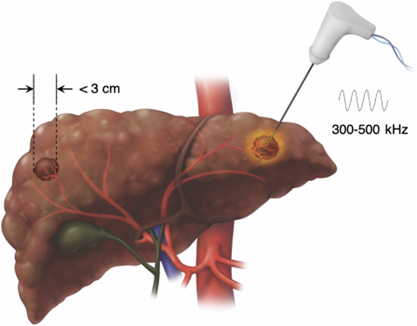
Figure 4: Radiofrequency ablation to treat HCC
MWA is different from RFA, in that no actual current passes through patient since energy is emitted via ablation antenna as microwaves (Figure 5). One of the first studies (phase II trial) with patients with unresectable primary or metastatic liver cancer being treated with MWA was published in 2007 demonstrating 47% alive after 19 months with no evidence of disease, no procedure-related deaths, and an overall mortality rate of 2.3%. The benefit of MWA compared to RFA is it does not suffer from “electrical sink” effect of RFA, allowing for ablation of tumors adjacent to large blood vessels. A 2018 phase II randomized controlled trial comparing MWA vs RFA in HCC showed similar outcomes amongst the two (6% vs 12% tumor progression, RR 1.62, 95% CI 0.66-3.94, p = 0.27) with infrequent complications and no treatment-related deaths.
Cryoablation is another form of local ablation that utilizes a cooling rod (probe) equipped with liquid nitrogen or argon to rapidly freeze tumor. This form is especially useful when the tumor is adjacent to a “heat sink” area like the inferior vena cava or other critical structures. Cryoablation suffers from adverse effects such as “cryoshock” with an incidence of 1% and risk of mortality of up to 28%, hemorrhage (<13%), and bile fistula/biloma (<10%).
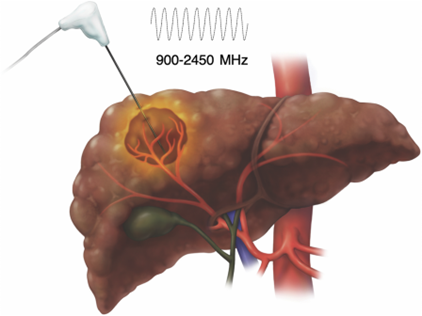
Figure 5: Microwave ablation to treat HCC
Steoreotactic body radiotherapy (SBRT) has gained significant importance as HCC is radiosensitive. It is recommended by the most recent AASLD practice guidelines as an alternative therapy to thermal ablation for patients with BCLC stage A HCC who are not candidates for surgical resection, including those with tumors >3 cm in size. SBRT is unique in delivering a high dose of radiation in fewer sessions (typically five sessions or less) which is convenient for patients. SBRT is different from transarterial radioembolization (TARE) in that it delivers radiation from outside the body (Figure 6). SBRT has been demonstrated both as first-line in treatment of HCC and as well as bridge therapy in advanced HCC. The 2022 multicenter RTOG1112 Trial evaluated SBRT followed by sorafenib vs sorafenib alone in patients with locally advanced HCC found higher survival in the former, with a trend towards statistical significance (15.8 vs 12.3 months, p =0.055).
The concept of SBRT is delivering highly conformal radiation dose distributions under image guidance to spare unaffected portions of liver as much as possible. However, radiation-induced liver disease (RILD), a veno-occlusive disease, is a significant complication of SBRT and is manifested by dysregulated hepatic function and jaundice with a case fatality rate as high as 5-13%.
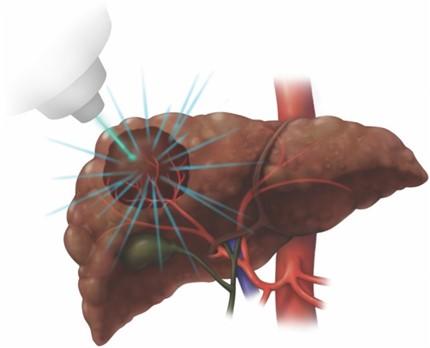
Figure 6: Stereotactic body radiotherapy (SBRT) to treat HCC
Chemoembolization or Radioembolization:
Transarterial chemoembolization (TACE) involves selective injective of a chemotherapeutic agent (doxorubicin, cisplatin, or mitomycin) mixed with a contrast agent in combination with arterial embolization. It can also be combined with drug eluting beads (DEB) where a hydrogel is loaded with chemotherapeutic agent, allowing for a higher dose, prolonged exposure time, and subsequently significant reduction in serious liver toxicity, per the results of the PRECISION V study. TACE is offered as a primary treatment option for patients with BCLC stage B HCC. Sensitivity analyses in a meta-analysis of 14 studies showed a significant benefit of chemoembolization with cisplatin or doxorubicin (OR 0.42, 95% CI 0.20 – 0.88) and treatment induced objective responses in 35% of patients (range 16 – 61%). Although TACE is not curative, it can be used as a neoadjuvant therapy or downstaging prior to possible LT.
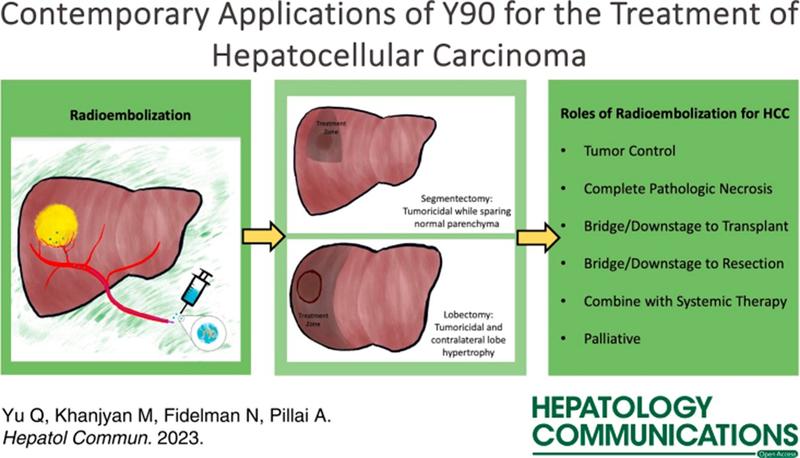
Transarterial radioembolization (TARE), also known as selective internal radiation therapy (SIRT), involves delivery of microspheres loaded with a radioisotop (e.g. yttrium-90, 90Y). There is a greater diffusion of administered agents in tumors with TARE than TACE or other techniques (see imaging effects in Figure 7 and Figure 8). A phase 2 randomized controlled trial of patients with HCC BCLC stages A or B found TARE to significantly prolong time to progression (TTP) compared to TACE (>26 months vs 6.8 months, p.= 0.0012).
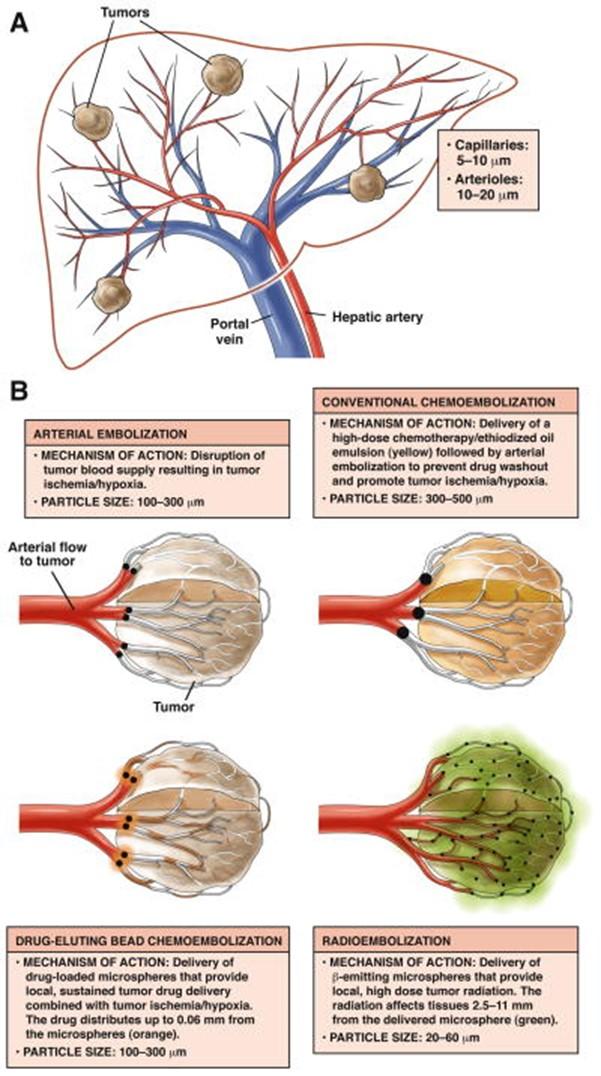
Figure 7: Mechanism of action of bland, chemoembolization, drug-eluting beads, and radioembolization in HCC
Figure 8: Contemporary applications of Y90 in treatment of HCC
Chemotherapy and immune checkpoint inhibitors:
Systemic therapy is recommended for patients with advanced stage HCC (BCLC stage C and beyond) or some intermediate stage (BCLC B) patients who progress despite locoregional therapies. Sorafenib, a tyrosine kinase inhibitor (TKI), first showed promise in a phase II study of 137 patients with a median survival of 9.2 months, partial response in 2.2% of patients, and stable disease in 33.6% of patients at 16 weeks. A phase 3 trial (SHARP trial) published in 2008 showed median overall survival of 10.7 months in sorafenib groups versus 7.9 months in placebo group, p < 0.001.
It took 10 years before the REFLECT trial was published, a phase 3 non-inferiority study of lenvatinib, an oral TKI targeting vascular endothelial growth factor (VEGF), vs sorafenib. REFLECT established lenvatinib was non-inferior to sorafenib with median survival time 13.6 months vs 12.3 months.
The study was followed by the open-label, randomized phase 3 IMbrave150 trial, which studied the combination of atezolimab, immunotherapy targeting programmed death-ligand 1 (PD-L1), and bevacizumab, monoclonal antibody targeting VEGF, in patients with unresectable HCC. The study included 336 patients in the study group compared to 165 patients in the comparison (sorafenib). Overall survival and progression-free survival was improved in atezolimab-bevacizumab compared to sorafenib (67.2% vs 54.6% at 12 months and 6.8 months vs 4.3 months, p < 0.001).
The phase 3 HIMALAYA trial studied the last set of first-line systemic therapy we have available: tremelimumab, cytotoxic T-lymphocyte associated antigen-4 (CTLA4) inhibitor, and durvalumab, PD-L1 inhibitor, against sorafenib. The treatment arm demonstrated improved survival (16.4 vs 13.7 months) compared to sorafenib. This is a viable therapy for patients who cannot tolerate bevacizumab due to increased risk of bleeding.
There are second-line options including the multikinase salvage regorafenib (also used in treatment of advanced gastrointestinal stromal tumors) and multiple ICIs (nivolumab + ipilimumab or pembrolizumab) that can be offered; however we will not discuss them in detail in this post. A simplified algorithm to follow can be seen in Figure 9.
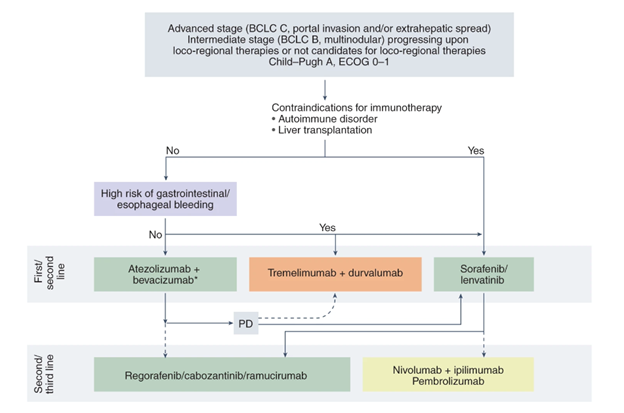
Figure 9: Systemic therapy in HCC – an algorithm
What is multidisciplinary care?
In the last four decades, the treatment of HCC has transformed from primarily surgical management to MDC. Locoregional and systemic multi-kinase therapies have entered the fold, offering more options for therapy depending on degree of liver dysfunction, tumor burden, and performance status. However, this has complicated management decisions that previously could be made by the sole hepatologist or surgeon. A multidisciplinary approach respects the invaluable expertise of multiple specialists involved in caring for a patient with HCC: hepatologists and hepatobiliary surgeons, transplant surgeons, diagnostic and interventional radiologists, medical and radiation oncologists, pathologists, palliative care physicians, nutritionists and dietitians, nurses, and social workers.
In addition to individualizing management decisions for each patient, an MDC model leverages discussion amongst different disciplines to make a meaningful difference in care. A Johns Hopkins study of 343 patients with HCC evaluated the effect of a single-day multidisciplinary liver clinic and found change in diagnosis in 8% of patients, alteration in interpretation of imaging in 18% of patients, and change in management plan in 42% of patients. A meta-analysis of 12 studies spanning 15,365 patients with HCC stratified outcomes by MDC status to show a significant improvement in overall survival (HR 0.60, 95% CI 0.45 – 0.88). Given this data, it is unsurprising that AASLD guidelines strongly recommend discussion and management of patients with HCC in an MDC setting.
Wrapping up:
HCC is a deadly disease, with a relatively poor prognosis compared to other malignancies despite decades of research. Part of it is the fear of developing therapies that could potentially worsen already poor hepatic function in patients with concurrent cirrhosis, however we have more management options now than ever in HCC history. We have come a long way from surgical resection as being the only therapy one century ago. Management options are now multifaceted and multilayered, which mandate a multidisciplinary approach. MDC is not only necessary to incorporate perspectives from clinicians, nursing, social work, and palliative care into a unified decision, but also improve survival.
I hope you enjoyed this Why series and that it will help you appreciate a bird’s eye view of HCC multidisciplinary care and current management options.
Take Home Points:
- Multidisciplinary care is an evidence-based, patient-centered approach to HCC care in the setting of numerous forms of therapies delivered by a diverse background of patient care teams
- Barcelona Liver Clinic Center (BCLC) staging criteria is recommended by society guidelines as a framework to guide therapy for patients with HCC by staging
- In addition to the well-known surgical resection and liver transplantation, treatment options for HCC include locoregional therapies (local ablation, chemoembolization, radioembolization, bland embolization), chemotherapies, and immune checkpoint inhibitors