Why is it important to think about non-cirrhotic portal hypertension?
Why is it important to think about non-cirrhotic portal hypertension?
Let’s start with a case!
A 25 yo with history of Common Variable Immunodeficiency (CVID) on monthly IVIG presents to the emergency room with hematemesis, and quickly goes into hemorrhagic shock. She is resuscitated, started on PPI and undergoes EGD, which shows large esophageal varices with white nipple sign and blood in the stomach. Varices are treated with esophageal variceal ligation, and she is started on octreotide and weaned off the pressors. She subsequently undergoes trans-jugular liver biopsy and HVPG measurement, which reveals no cirrhosis, only thickening and occlusion of the portal veins in some portal triads, and incomplete septal fibrosis. HVPG is measured at 5 mmHg.
There is evidence of portal HTN, as the patient has large esophageal varices, however the HVPG measurement was normal. How is this possible? Isn’t measurement of the hepatic venous pressure gradient (HVPG) the gold standard technique for evaluation of portal hypertension?
How is HVPG measured?
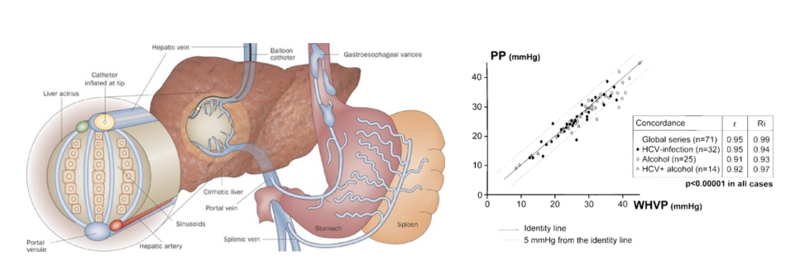
To better understand why this might be, we need to understand how HVPG is measured. Under local anesthesia, the right jugular vein is catheterized, a venous introducer is placed, and a balloon-tipped catheter is advanced under fluoroscopic control into the inferior vena cava and a hepatic vein. The procedure is usually performed by interventional radiology.
First, the Free Hepatic Vein Pressure (FHVP) is measured by placing the tip of the catheter 'freely' in the hepatic vein—the FHVP should be similar in value to the inferior vena cava pressure; a difference of >2 mmHg signifies that the catheter is probably inadequately placed or that a hepatic vein obstruction exists. Then, the wedged hepatic venous pressure (WHVP) is measured by occluding the hepatic vein, usually by inflating a balloon at the tip of the catheter. Adequate occlusion of the hepatic vein is confirmed by slowly injecting contrast dye into the vein. Therefore, WHVP is a measure of hepatic sinusoidal pressure, not the portal pressure.
In cirrhosis, WHVP gives an accurate estimate of portal pressure, given the liver is stiff, and the sinusoids have lost their connections. This has been demonstrated both for alcohol-associated and viral cirrhosis. The study included patients with viral or alcohol related cirrhosis undergoing TIPS placement, during which portal vein puncture is done to create the shunt. Therefore, portal pressure, and subsequently portal pressure gradient (PPG = FHVP – PP) can be reported. During this study, direct portal pressure measurement was obtained and compared with WHVP. As you can see in the graph below, PP and WHVP had a very high correlation (r > 0.9, p <0.00001).
However if the site of the portal hypertension is before the sinusoids, then HVPG measurement is not able to identify the increased in pressure gradient. In pre-sinusoidal poral hypertension, PP is elevated, however it is not reflected by WHVP. Since HVPG is the difference between WHVP and FHVP, it is unable to capture the increased pressure gradient in this situation. As you can see, HVPG measurement is going to miss the pre-sinusoidal causes of portal HTN. So a patient with normal HVPG, can indeed have portal hypertension.
Figure 1: In the cirrhotic liver, the pressure of the static column of blood created by balloon inflation cannot be decompressed at the sinusoidal level owing to disruption of the normal intersinusoidal architecture. Therefore, WHVP equilibrates with and is equivalent to portal pressure.
What are the cases of increased portal pressure gradient?
It is important to remember that whenever you have a patient with portal hypertension without cirrhosis, think about non-cirrhotic portal HTN!
Portal hypertension is defined as an increase in portal pressure gradient (PPG), that is, the pressure difference between the portal vein and the inferior vena cava. This increase in PPG, can occur as pre-hepatic, intra-hepatic, and post-hepatic. Cirrhosis accounts for more than 90% of the cases with portal hypertension, and is categorized as intra-hepatic portal hypertension. All other causes of portal hypertension in the absence of cirrhosis are included in a broad category called “non-cirrhotic” portal hypertension. The table below summarizes the majors sites of increase in portal pressure gradient and provides some examples for each category. In all of these disorders, the management of portal HTN complications, such as ascites and variceal bleeding, is the same as the portal HTN complications caused by cirrhosis, and follows the same set of guidelines.
|
Examples |
|
|
Prehepatic |
Splenic vein thrombosis Portal vein thrombosis Extrinsic compression of splenic/portal vein Arteriovenous fistula |
|
Hepatic (Presinusoidal) |
Schistosomiasis Sarcoidosis Congenital hepatic fibrosis Small bile duct disease (PBC, small duct PSC) Porto-sinusoidal vascular disorder (PSVD) |
|
Intrahepatic (Sinusoidal) |
Sinusoidal fibrosis (methotrexate, alcohol, vinyl chloride) Amyloidosis Infiltration (mastocytosis) Gaucher disease Hepatocyte enlargement (acute fatty liver) Cirrhosis |
|
Intrahepatic (Postsinusoidal) |
Sinusoidal obstruction syndrome/Veno-occlusive disease (VOD) |
|
Posthepatic |
Hepatic vein thrombosis (Budd-Chiari syndrome) IVC membrane or thrombus Constrictive pericarditis |
Why is it important to think about porto-sinusoidal vascular disorder (PSVD)?
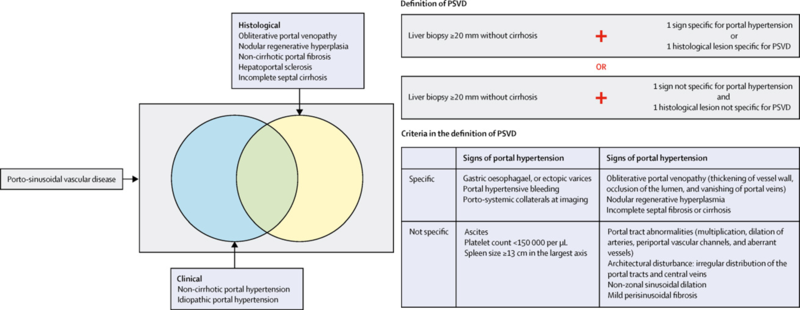
In this “why series” we are going to exclusively focus on one type of non-cirrhotic portal HTN named Porto-sinusoidal vascular disorder (PSVD). PSVD was first proposed by the Vascular Liver Diseases Interest Group (VALDIG) group as a new terminology for a unique disease entity in 2017 to describe a heterogenous group of conditions, causing non-cirrhotic portal hypertension that mainly involve the portal venules or the pre-sinusoidal areas (Panel 1). This entity has numerous names in different countries such as “non-cirrhotic portal fibrosis” (in India), “idiopathic portal hypertension” (in Japan), “hepatoportal sclerosis” (in Western countries), along with many other names such as “non-cirrhotic intrahepatic portal hypertension”, “nodular regenerative hyperplasia”, and “obliterative portal venopathy”.
Panel 1A. Elements defining porto-sinusoidal vascular disease
Panel 1B. Definition of porto-sinusoidal vascular disease for clinical practice
In addition, the patient needs to have either signs of portal hypertension or histologic features of PSVD (Panel 3). High clinical suspicion is needed in practice, to correctly identify these patients. Clinical signs of portal HTN but with low liver stiffness measurement and completely normal synthetic function, or presence of known etiological factors (panel 4), should raise suspicion for these conditions, and prompt a liver biopsy. HVPG measurements in this disorder will be normal, as it measures the difference between hepatic vein pressure and sinusoidal wedge pressure.
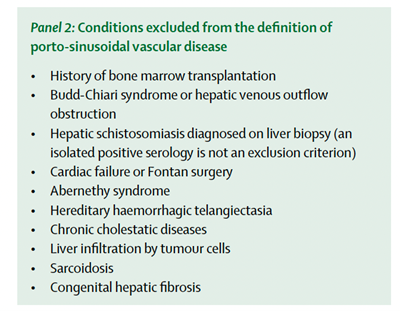
A good quality liver biopsy to rule out cirrhosis is essential before establishing the diagnosis of PSVD. In addition to cirrhosis, there are other conditions such as sarcoidosis, congenital hepatic fibrosis, schistosomiasis, that are excluded from this definition (panel 2).
The presence of common causes of liver disease such as viral, alcohol, or metabolic syndrome, or the presence of portal vein thrombosis (PVT) does not rule out PSVD, as they can coexist. However there are specific conditions that are excluded from this definition (panel 2). In addition, the presence of portal HTN is not necessary for diagnosis of PSVD, but it is important to note that patients with histological evidence of PSVD but without any evidence of portal HTN, are not at risk for decompensation or liver related mortality.
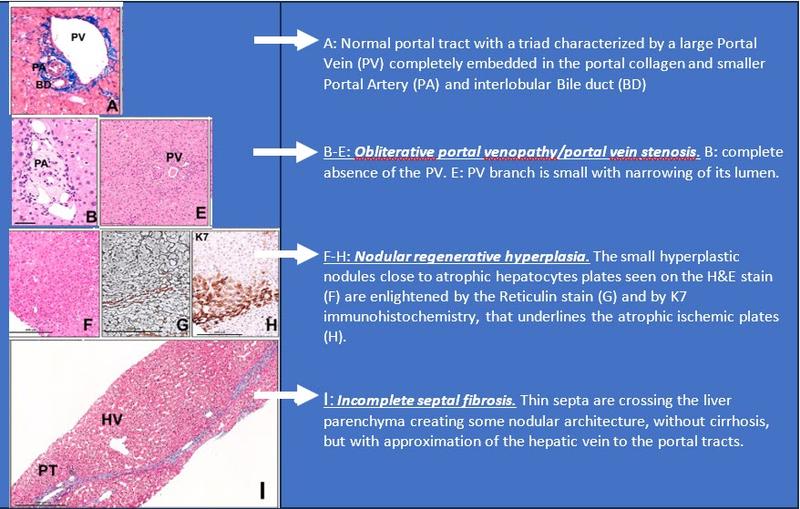
Panel 3: Histological features of PSVD
Are there non-invasive ways to diagnose PSVD?
Although for diagnosis of PSVD, a good quality liver biopsy is necessary, in clinical practice, there are non-invasive tools to suspect presence of PSVD.
In PSVD, Liver stiffness measurement (LSM) does not correlate with degree of portal hypertension. However, spleen stiffness measurements (SSM) using shear wave elastography are markedly increased in PSVD.
In patients with signs of portal hypertension, high SSM and normal or slightly elevated LSM should prompt further investigations to rule out PSVD. A recent study showed that SSM/LSM AUROC was 0.940 (p<0.001) for differentiating PSVD from cirrhosis. The study proposed 2 as a cut-off, with good sensitivity (86.5%), specificity (92.7%), and accuracy (89.7%) for the diagnosis of PSVD. SSM has also been used to predict high risk varices in patients with PSVD. A recent study showed SSM of > 35.4 kPa has 93% sensitivity, 60% specificity, and 91% negative predictive value in diagnosis of high-risk varices in patients with PSVD. However further studies are needed to externally validate these findings.
In addition to shear wave elastography, there are other clues on imaging that can point towards presence of PSVD. On cross sectional imaging, liver surface is smooth and the surface nodularity, which is a typical feature of cirrhosis, is not present in majority of patients with PSVD. Only 16% of patients with PSVD have a nodular liver surface on imaging. Atrophy of segment IV and hypertrophy of segment I are more commonly seen in cirrhosis compared to PSVD (84% vs 28%), while right lobe atrophy, and caudate lobe hypertrophy is more characteristic for PSVD. Signs of portal hypertension such as splenomegaly and porto-systemic collaterals are more severe in PSVD compared to patients with cirrhosis and similar liver function. In addition, “ectopic” collaterals and fundal varices are more frequent in PSVD.
How is PSVD managed?
Once the diagnosis of PSVD is made, patients should be screened for associated immunological diseases, pro- thrombotic or genetic disorders and exposure to drugs/ toxins (Table 1). Patients need to undergo EGD for variceal screening, and the non-invasive criteria proposed by BAVENO VII does not apply. Management of varices are similar to cirrhosis guidelines with non-selective beta-blockers or esophageal variceal ligation, and small studies have shown similar outcomes. Doppler ultrasound every 6 months in patients who have features of portal HTN is recommended, as the yearly probability of developing PVT is about 9%. There is no proven role for prophylactic anticoagulation, and in patients who develop PVT, it is managed based on cirrhosis PVT management guidelines.
TIPS can be considered to treat severe complications of portal hypertension. These patients have excellent outcomes if they have normal kidney function and do not have severe extrahepatic comorbidities. Patients with PSVD have fewer complications related to TIPS compared to patients with cirrhosis. In a study comparing these 2 groups following TIPS insertion for management of variceal bleeding, patients with PSVD had significantly lower rates of hepatic encephalopathy, hepatic impairment, and long-term mortality.
Despite most patients having preserved liver function, some progress to require liver transplant, mainly related to decompensations (refractory ascites, hepatic encephalopathy, hepatopulmonary syndrome). A recent study of 79 patients with LT for PSVD, showed 82.2%, 80.7%, and 68.6% survival at 1, 2, and 5 y, respectively. Although rare, PSVD recurrence is possible after LT. In the above series, 3 had recurrence after transplant.
So back to our case! She has PSVD, as evidenced by portal hypertension (esophageal variceal bleeding) with the absence of cirrhosis. Underlying diagnosis of CVID is the likely etiological factor putting her at risk for PSVD. She will need to get treatment with NSBB and EVL (secondary prophylaxis), and undergo duplex US every 6 months to screen for PVT.