Policy Communications
AASLD addresses a wide range of policies that affect researchers, clinicians, and patients, by focusing on issues critical to the science and practice of hepatology. In our advocacy work, we engage with Congress and members of the executive branch to influence legislation and implement federal programs to advance AASLD's core missions. The Public Policy Committee informs these efforts by prioritizing issues of importance, providing subject matter expertise, and playing an active role in AASLD’s advocacy activities.
Share Your NIH Stories
We are collecting stories of how NIH funding has impacted your career, and how changes at NIH are impacting you and your research, especially in the areas of social disparities of health and health equity. These stories will be shared with key stakeholders as we advocate for the NIH. To share your story, click here.
Policy Communications Updates
Congress finalizes Fiscal Year 2026 Health Funding After Brief Shutdown
On Feb. 3, President Donald Trump signed the House-passed six-bill spending package to fund the Departments of Health and Human Services (HHS) and most other agencies for the remainder of Fiscal Year 2026. Congress had been on track to finalize spending levels in advance of the Jan. 30 expiration of the continuing resolution (CR). Following Immigration and Customs Enforcement (ICE) actions in Minnesota, democrats negotiated a two-week CR to fund DHS until February 13 and to provide for the development and adoption of a legislative package of ICE reforms. Because the House was in recess when the Senate adopted the revised funding package, a brief partial government shutdown occurred.
Funding highlights in the FY 2026 Labor, Health and Human Services, Education, and Related Agencies (LHHS) bill include:
- $48.7 billion for the National Institutes of Health (NIH), which includes funding from the 21st Century Cures Act, the Public Health Service (PHS) Evaluation Act, and the Advanced Research Projects Agency for Health (ARPA-H). This represents an increase of $415 million from FY 2025 levels.
- This includes $7.35 billion for the National Cancer Institute (NCI), an increase of $128 million from FY 2025; $2.33 billion for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), an increase of $16 million; $6.6 billion for the National Institute of Allergy and Infectious Diseases (NIAID), an increase of $23 million, and $595 million for the National Institute on Alcohol Abuse and Alcohol-Associated Disorders (NIAAA), flat-funded from FY 2025.
- The legislation includes language that prohibits NIH from using the multi-year funding mechanism more in FY 2026 than in FY 2025; 39 percent of grants were supported by the multi-year funding mechanism in FY 2025. It also prohibits the administration from making changes to the cap on indirect costs.
- $1.5 billion for the Advanced Research Projects Agency for Health (ARPA-H), flat-funded from FY 2025.
- $9.2 billion for the Centers for Disease Control and Prevention (CDC), a decrease of $19 million
- This includes $1.38 billion for HIV/AIDS, Viral Hepatitis, Sexually Transmitted Diseases, and Tuberculosis Prevention, a decrease of $7 million from FY 2025; and
- $46 million for the Division of Viral Hepatitis, an increase of $3 million from FY 2025
Thank you to all AASLD members for your advocacy, and we look forward to your continued support as the FY 2027 appropriations process begins.
CMS Announces Third Round of Medicare Drug Price Negotiation: Liver Cancer Treatment Included
The Centers for Medicare & Medicaid Services (CMS) has released the list of drugs subject to the third cycle of Medicare drug price negotiation under the Inflation Reduction Act. Lenvima, used to treat liver cancer, is included on the list.
This follows last year's negotiation cycle in which Xiafaxan, used to reduce the risk of hepatic encephalopathy in patients with cirrhosis, was included. The negotiated price of $1,000 for Xiafaxan will be applicable in the Medicare program in 2027.
The negotiated prices for Lenvima and the 14 other drugs included in the third negotiation cycle will be announced at the end of this year and will take effect in 2028.
AASLD will continue to monitor the implementation of the IRA’s drug pricing and keep members updated as information becomes available.
WHO Considers Expanding NCD Definition to Include Liver Disease
The World Health Organization (WHO) is considering a proposal to expand its global definition of noncommunicable diseases (NCDs) to formally include liver disease. This is an important step toward elevating liver health within global public health policy and practice.
For the global hepatology community, this development reflects decades of scientific evidence demonstrating that liver diseases are among the leading causes of preventable illness and death worldwide. Conditions such as metabolic dysfunction–associated steatotic liver disease (MASLD), viral hepatitis, and alcohol-associated liver disease are closely interconnected with other major NCDs, including diabetes, cardiovascular disease, and obesity.
Inclusion within the NCD framework would strengthen global surveillance, elevate recognition of liver disease within national health agendas, and accelerate the integration of liver health into prevention and primary care. Importantly, it reflects a growing understanding of the liver’s central role in whole-person, integrated care, supporting earlier identification of risk and disease across the lifespan, including in childhood.
AASLD welcomes this momentum and will continue to engage with international partners, policymakers, and the global hepatology community to advance evidence-based policies that prioritize liver health, support early detection and prevention, and improve outcomes for people living with liver disease worldwide.
Honoring Black History Month Through Liver Health Advocacy
Public policy is critical to addressing liver health disparities and outcomes that disproportionately affect Black communities, including access to prevention, early diagnosis, and equitable treatment for liver disease. Evidence-based policy is essential to expanding access to vaccination, screening, early diagnosis, treatment, and ultimately, saving lives.
Did You Know
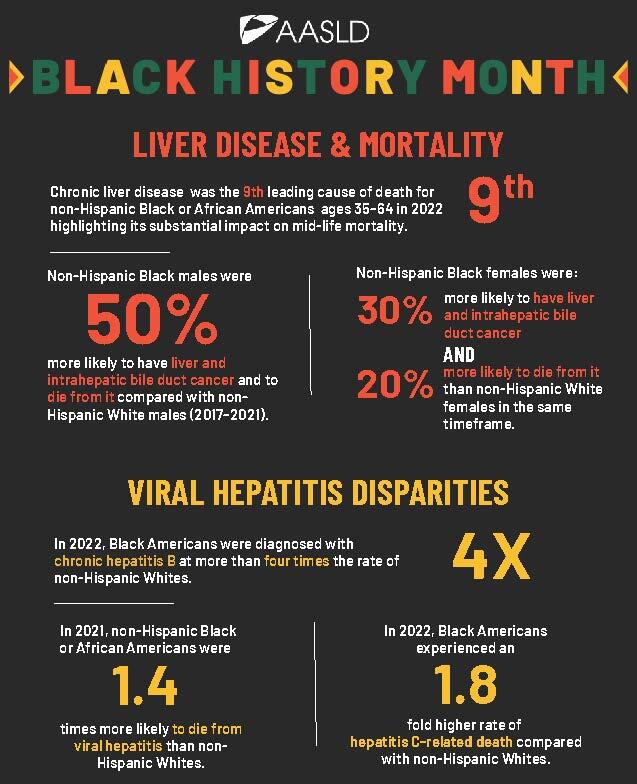
AASLD actively advocates for policies that strengthen hepatitis prevention and elimination efforts, protect access to lifesaving vaccines and treatments, and ensure that science guides decision-making.
Stay engaged on AASLD’s efforts to advance equity, protect public health, and move us closer to elimination of viral hepatitis. Use the Policy Action Center to contact your elected officials on critical liver health and hepatitis-related issues.
AASLD Raises Concern Over Removal of Evidence-Based Alcohol Guidance in 2025–2030 Dietary Guidelines for Americans
On January 7, 2026, the Department of Health and Human Services (HHS) and the Department of Agriculture (USDA) released updated Dietary Guidelines for Americans (DGA) for 2025-2030, removing any specific guidance on alcohol consumption. The updated guidelines instead advise that people should “consume less alcohol for better overall health” and “limit beverages,” without recommending clear limits. The guidelines also outline broad, incomplete categories of people that should “completely avoid alcohol” or be “mindful of alcohol consumption.”
The American Association for the Study of Liver Diseases (AASLD) expresses deep concern that the revised DGA omit specific guidance related to alcohol consumption. Earlier editions of the DGA provided clear daily limits for alcohol consumption based on available evidence. In contrast, the 2025–2030 guidelines do not establish any daily limits and do not account for biological differences in alcohol metabolism between men and women.
While AASLD agrees that broadly consuming less alcohol supports better overall health, the American public deserves clear recommendations based on the best available evidence to guide their consumption choices. These guidelines fall short in this respect and deny Americans evidence-based recommendations to make healthy choices.
In 1990, the DGA recommendations were updated to distinguish alcohol consumption limits for men and women. These daily limits were based on data showing that alcohol consumption of more than two drinks per day for men and more than one for women has significant impacts on health. Recent research examining limits on alcohol consumption includes data showing that the previous DGA limits may need to be lowered.
Additionally, the updated guidelines are silent on the link between alcohol and cancer. Twenty-five years ago, the guidelines first warned that alcohol increased the risk of breast cancer. Since that warning, alcohol research has shown a link between alcohol and at least seven types of cancer. The 2025 U.S. Surgeon General’s Advisory on Alcohol and Cancer Risk highlighted that alcohol use is a “leading preventable cause of cancer in the United States,” noting that it contributes to “nearly 100,000 cancer cases and about 20,000 cancer deaths each year.”
AASLD will continue to educate the public about the impact of alcohol consumption on liver health and other potential side effects, including pancreatitis, dementia, other tissue damage, cancer in the liver and in the rest of the body, cardiovascular disease, and social injuries such as motor vehicle accidents, workplace accidents, family disruption, and loss of mental health, despite these changes.
AASLD urges the administration to revisit its guidance on alcohol and restore clear, evidence-based recommendations so individuals have the tools they need to make informed decisions about alcohol and its impact on their overall health.
CDC Accepts Recommended Changes to the Hepatitis B Birth Dose Vaccine Schedule
Earlier in December, the CDC’s Advisory Committee on Immunization Practices (ACIP) voted to roll back its longstanding recommendation for the universal hepatitis B birth dose, despite decades of evidence showing the vaccine prevents up to 95% of new infections and saves thousands of lives each year.
Unfortunately, ACIP’s recommendation was accepted by Acting CDC Director Jim O’Neill on Dec. 16.
Under the revised recommendation, infants born to mothers who test negative for hepatitis B will no longer receive the vaccine at birth, delaying the first dose until two months of age. This change increases the risk of preventable chronic hepatitis B infections, liver cancer, and missed vaccination opportunities, particularly in communities already facing higher disease burden and gaps in access to care.
AASLD has been a strong, vocal advocate for retaining the birth dose. In the months leading up to the December vote, AASLD engaged policymakers and coordinated closely with partner societies to underscore the clinical and public health risks of changing the birth dose policy. Following the ACIP vote, AASLD, in partnership with ACG, AGA, ASGE, IDSA, and NASPGHAN, released a joint statement that opposed the reversal and urged HHS and CDC leadership to reject ACIP’s recommendation. Read our full statement here.
Despite the outcome, our member advocacy made a powerful impact. Nearly 1,200 letters were sent to representatives through AASLD’s Policy Action Center on this issue alone, making this our most engaged advocacy alert of 2025. Your voices elevated the clinical evidence, highlighted patient impact, and ensured policymakers heard directly from the hepatology community. Thank you for speaking up.
AASLD will continue advocating for the hepatitis B birth dose and for policies that protect patients, science, and public health. We will keep members informed as this issue moves forward.
AASLD Statement: Supporting Universal Hepatitis B Birth-Dose Vaccination
As the leader in hepatology, AASLD is committed to advancing evidence-based policies that protect liver health. Vaccination against hepatitis B at birth is one of the most effective tools we have to prevent lifelong infection and reduce liver disease, including liver cancer. By protecting infants from hepatitis B infection, we can prevent liver disease, reduce liver cancer, and improve lives across the U.S.
The CDC’s universal hepatitis B birth-dose recommendation is a proven public health success. Since it was introduced in 1991:
- Infant hepatitis B infections have declined 95%,
- More than 90,000 deaths prevented, and
- More than 6 million infections and nearly 1 million hospitalizations prevented in less than 30 years.
These statistics are not just numbers. They shielded a generation from preventable disease.
Currently, the universal birth-dose recommendation is under review. Weakening or altering this policy could create inconsistencies in vaccine access, leaving thousands of infants vulnerable.
AASLD stands ready to work with legislators, policymakers, and public health partners to ensure that science-based, evidence-driven guidance remains at the forefront of public health decisions. Protecting newborns from hepatitis B is a national public health priority.
Together, we can safeguard the next generation from preventable liver disease and continue the remarkable progress achieved over the past three decades.
CMS Releases CY 2026 Medicare Physician Fee Schedule Proposed Rule
Last month, the Centers for Medicare & Medicaid Services (CMS) released the Calendar Year (CY) 2026 Medicare Physician Fee Schedule (MPFS) proposed rule, outlining key updates that impact physician payment, policy, and quality reporting. This rule updates payment policies and payment rates for Part B services furnished under the MPFS and makes changes to the Quality Payment Program.
The proposed rule includes:
- Dual conversion factors for the first time: This includes $33.59 for qualifying advanced APMs (+3.83%) and $33.42 for non-qualifying APMs (+3.62%). These reflect a 2.5% increase adopted by Congress.
- Major changes to practice expense methodology: CMS now proposes unequal allocation of indirect costs, favoring office-based settings over facility-based ones. The proposed change would result in significant decreases in the values of services, including evaluation and management services, performed in the facility setting.
- Efficiency adjustment proposal: A -2.5% efficiency adjustment to work RVUs for certain procedures, radiology services, and diagnostic tests, reflecting gains from clinical experience and technology. Exempt: E/M and time-based services. This adjustment would be updated every 3 years.
- New hepatology measures: CMS proposed to add a new measure to the Merit-Based Incentive Payment System: Hepatitis C Virus (HCV): Sustained Virological Response (SVR). If finalized, this measure will be available to report beginning in 2026.
The press release and fact sheet are also available for review.
AASLD is reviewing the proposed rule and will submit comments before the Sept. 12 deadline.
Threats to Hepatitis Vaccines Emerge in HHS: July 18, 2025
Department of Health and Human Services (HHS) Secretary Robert F. Kennedy, Jr. dismissed the entire membership of the Advisory Committee on Immunization Practices (ACIP) in early June and promptly appointed eight new members – some of whom are critics of vaccine science – in advance of their scheduled meeting on June 25-26. Secretary Kennedy claimed that the turnover was necessary to re-establish public confidence in vaccine science.
ACIP, a committee of nonfederal experts, makes recommendations to HHS regarding the use of vaccines and related agents for the control of vaccine-preventable disease in the United States. Besides setting the childhood and adult immunization schedules, they also review and make recommendations on the annual flu and COVID vaccines based on many factors, including the safety and effectiveness of the vaccine. Vaccine coverage requirements, including for those currently offered at no cost, are tied to ACIP’s recommendations.
This shakeup has implications for the hepatitis vaccines. ACIP previously recommended that the hepatitis B vaccine be administered to all infants at birth, unvaccinated children younger than 19 years of age, adults 19-59 years of age, and adults aged 60 years and older with risk factors for hepatitis B or without identified risk factors but seeking protection. AASLD worked closely with Hepatitis Caucus Co-chairs Representatives Hank Johnson (D-GA) and Nydia Velazquez (D-NY) who sent a letter to Secretary Kennedy urging him not to alter the vaccine schedule for hepatitis vaccines.
At the recent ACIP meeting, it was announced new work groups are being created to study the cumulative effects of the childhood and adolescent vaccine schedules, including the hepatitis B vaccine. AASLD will advocate to ensure Americans have access to these vaccines, which are critical to reducing the burden of cirrhosis and liver cancer.
AASLD Leaders Advocated on Capitol Hill—Join Us by Taking Action Today
On June 17, AASLD President Dr. Grace Su, joined by CEO Matthew D’Uva and AASLD staff, visited Capitol Hill to advocate for sustained and robust federal investment in the National Institutes of Health (NIH). This visit comes at a critical time, as the House and Senate Appropriations Committees prepare to allocate funding for the NIH and other key health programs later this summer.
Dr. Su met with influential members of both the Senate Appropriations Committee and the Senate Health, Education, Labor, and Pensions (HELP) Committee, emphasizing the NIH’s pivotal role in advancing research and treatment for chronic liver disease. She highlighted transformative breakthroughs made possible by NIH funding, encompassing a curative therapy for hepatitis C, advancements in liver transplantation, and support for promising research careers in hepatology, including her own.
Her message was especially timely in light of President Trump’s proposed FY 2026 budget, which includes a 40% cut to NIH funding and a major restructuring of the agency. While the administration's budget signals the official start of the annual appropriations process, it is ultimately Congress that determines the final funding levels.
By engaging directly with lawmakers, Dr. Su underscored AASLD’s commitment to ensuring robust federal support for medical research to fuel innovation, save lives, and drive the future of liver disease care.
Join AASLD in taking a stand for liver health. Oppose harmful cuts to Medicaid and student loan programs, and urge Congress to support the Cure Hepatitis C Act. Contact your legislators now through AASLD’s Policy Action Center.
Statement of Support and Call for Action: Cure Hepatitis C Act of 2025
The American Association for the Study of Liver Diseases (AASLD) applauds Senators Bill Cassidy (R-LA) and Chris Van Hollen (D-MD) for their bipartisan leadership to eliminate hepatitis C. The Cure Hepatitis C Act of 2025 represents a unique opportunity for Congress, the Department of Health and Human Services, and the health care community to partner to cure a devastating virus while saving over $6 billion in the 10-year budget window.
We urge AASLD members to show your support for the bill by contacting your senators to advocate for its passage. AASLD will be actively advocating for the legislation’s adoption beginning on Tuesday, June 10 at a congressional briefing. More details are available here.
The Hepatitis C Elimination Program authorized by this legislation puts the necessary pieces in place to ensure that the 2.2 million people in the United States who are chronically infected with hepatitis C will not see the virus progress to cirrhosis, liver cancer, and potentially even death. The legislation creates an innovative purchasing model and establishes the infrastructure necessary in communities across the country to support a test-to-treat program to ensure that more people are diagnosed and linked to treatment at no cost. Additionally, it provides multiple opportunities for AASLD and other stakeholders to inform the implementation of the program—which is critical for ensuring that those working in these communities best understand what resources and infrastructure are needed to test and treat those living with the virus.
“Despite the availability of a curative therapy for over 10 years, people are still dying from hepatitis C. We must seize this opportunity to get these effective therapies to those affected. I am confident that this legislation will facilitate that. AASLD stands ready to work with Senators Cassidy and Van Hollen to pass this legislation as quickly as possible to prevent more unnecessary deaths from this virus,” said AASLD President Grace Su, MD, FAASLD.
Elimination of viral hepatitis, including hepatitis C, is a top public health priority for AASLD. The society has partnered with federal and state governments and other stakeholders to achieve the goal of hepatitis B and C elimination by 2030. When the curative direct acting antivirals were approved 12 years ago, AASLD collaborated with the Infectious Diseases Society of America to develop evidence-based practice guidelines for the diagnosis, management, and treatment of hepatitis C, which are updated regularly. Additionally, the organization has served as a convenor to foster collaboration, exchange ideas, and increase knowledge translation on hepatitis C elimination.
This legislation demonstrates how investments in research and public health programs can deliver for the American people. Drs. Harvey Alter, Michael Houghton, and Charles Rice identified the hepatitis C virus as a bloodborne pathogen that causes acute and chronic hepatitis, cirrhosis, and liver cancer in 1989. Their discovery led to further studies supported by the National Institutes of Health that yielded information crucial for the development of today’s curative therapies. The next step is to get these treatments to those in need.
To support AASLD’s advocacy on this legislation, take action today. Contact your senators and urge them to cosponsor the bill.
Reconciliation Package Advances to the Senate Where Changes Expected
Before Memorial Day, the House advanced the One Big Beautiful Bill Act (H.R. 1), the Republicans’ reconciliation package that extends the 2017 tax cuts and provides funding for defense and homeland security while making cuts to Medicaid and other programs. The legislation advanced by a vote of 215-214. Representatives Thomas Massie (R-KY) and Warren Davidson (R-OH) were the only two Republicans to vote with the Democrats against the bill; they opposed the bill’s significant increase in deficit spending.
The legislation cuts approximately $700 billion from the Medicaid program in the next decade with roughly 7.6 million Americans expected to lose coverage. Specifically, the legislation does the following:
- Imposes mandatory Medicare work requirements with individuals between 19-64 years old required to demonstrate that they have worked, volunteered, or attended school for 80 hours each month with certain exceptions;
- Requires states to impose cost sharing of up to $35 per service on the expansion population with incomes 100-138% of the federal poverty level but explicitly exempts primary care, mental health, and substance use disorder services from cost sharing;
- Requires states to verify the eligibility of the expansion population every six months rather than once a year;
- Limits retroactive Medicaid coverage to one month prior to application for coverage;
- Prohibits states from establishing new provider taxes or increasing the amount of existing provider taxes, which is an avenue states use to increase their federal Medicaid match; and
- Reduces the expansion match rate from 90% to 80% for states that provide health coverage or other financial assistance using state funds to undocumented individuals.
Many of these provisions will be effective December 31, 2026.
Of note, the package includes an update to the Medicare Physician Fee Schedule conversion factor. In 2026, the legislation provides for an update equal to 75% of the Medicare Economic Index (MEI). Each year thereafter, the conversion factor update will equal 10% of MEI. The American Medical Association strongly supports this provision because it incorporates an MEI-based update in statute. However, the legislation does not address the 2.83% cut that was implemented on January 1 of this year, and the 2026 update would not be large enough to offset that cut leaving the 2026 conversion factor lower than that in 2024. Additionally, there are concerns that updates equal to 10% of MEI will not keep pace with inflation or address the stagnation in physician reimbursement that has occurred over the last two decades.
Moving forward, this bill will face many challenges and changes in the Senate, as Republican Senators such as Rand Paul (R-KY), Ron Johnson (R-WI), and Rick Scott (R-FL) have already voiced their criticism of the bill. Paul shared his concerns over the high increase to the debt ceiling and stated that he would only consider the bill without this increase, while Johnson and Scott are looking to find ways to reduce the federal deficits. There are many Republican Senators who have expressed concern about the Medicaid cuts in the House package, including Senators Josh Hawley (R-MO), Susan Collins (R-ME), and Lisa Murkowski (R-AK) are likely to have red lines around what—if any—cuts they will accept in Medicaid. Senate Majority Leader John Thune (R-SD) will have to navigate these and other issues over the next month plus to meet his stated goal of passing the legislation before July 4th.
President Transmits Preliminary Budget Request to Congress with Steep Cuts to Health Programs
The White House released its preliminary fiscal year (FY) 2026 budget request outlining deep cuts to health and other non-defense discretionary programs and more information about the reorganization of the Department of Health and Human Services (HHS). The request proposes to cut HHS by 26.2% to $93.8 billion from FY 2025 levels. Like all White House budget requests, this is a proposal, and the funding cuts will not become law unless Congress implements them during the appropriations process.
As included in the recently leaked FY 2026 passback memo, the budget formally requests $27 billion for research at the National Institutes of Health (NIH), a decrease of nearly $18 billion. Additionally, the budget “proposes to reform NIH and focus NIH research activities in line with the President’s commitment to MAHA, including consolidating multiple overlapping ill-focused programs into five new focus areas with associated spending reforms”. It specifically mentions a new National Institute on Body Systems Research, which will combine the National Institute of Diabetes and Digestive and Kidney Diseases, the National Heart, Lung, and Blood Institute, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and a National Institute on Behavioral Health, which will combine the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse and the National Institute of Mental Health, according to the passback budget. The budget does not include funding levels for these new institutes or the existing institutes and centers that will be retained. Neither the preliminary budget request nor the passback budget provides the scientific rationale for or details how these consolidations will work in practice. It is important to note that the administration has the statutory authority to reform NIH’s structure as is being considered. AASLD is monitoring these activities closely and will weigh in with the administration and Congress to ensure that the full spectrum of liver disease research continues to be supported.
Additionally, the budget proposes to consolidate funding for infectious disease and opioids, viral hepatitis, sexually transmitted infections, and tuberculosis programs into one grant program, which would be funded at $300 million, at the Centers for Disease Control and Prevention (CDC); this represents a $77 million cut below current funding levels for these programs. For the CDC, the President requests $5.6 billion, a 18.3% decrease below current funding levels. Also of note, the budget includes $500 million to build on the work of the President’s Make America Healthy Again Commission.
AASLD will continue to keep members updated on the FY 2026 budget and appropriations process and to advocate to ensure that federal programs that support liver disease research and treatment receive the highest appropriated funding levels possible.
AASLD Statement on HHS Reorganization
The American Association for the Study of Liver Diseases (AASLD) is extremely concerned that the recently announced cancellation of grants to state health departments and researchers and the reorganization of the Department of Health and Human Services (HHS) will undermine Secretary Robert F. Kennedy, Jr.’s goal to make Americans healthy again by eroding the public health infrastructure and access to care. Taken together, these policies decrease HHS’s expertise and experience to address existing public health challenges, like viral hepatitis and the country’s obesity epidemic, as well as future challenges.
The HHS reorganization, which reduces the number of HHS’s divisions from 25 to 12 and workforce by 20,000 through a reduction in force and early retirements, was completed without input from Congress, key stakeholders, and other health care and public health experts. The lack of transparency with which these changes were made make it difficult to assess how they will affect the government’s ability to protect the country’s health and safety. While AASLD supports efforts to improve the effectiveness of federal programs, we are concerned about the far-reaching ramifications of these changes. The loss of 1,200 staff at the National Institutes of Health and rescission of grant awards will affect the development and accessibility of future cures for the full range of liver diseases as well as the other chronic conditions burdening Americans’ health and discourage young investigators from pursuing careers in biomedical research. HHS states that the changes at CDC will allow the agency to better prepare and respond to outbreaks but does not address the fate of prevention and surveillance programs for conditions including viral hepatitis and cancer.
AASLD calls on HHS to pause these efforts and consult with Congress and other stakeholders on how to meet the administration’s goals while safeguarding and improving Americans’ health.
Take Action! Urge Congress to Address Chronic Liver Disease
Representatives Hank Johnson (D-GA), Don Bacon (R-NE), and Nydia Velazquez (D-NY) are leading a Dear Colleague letter to garner support for AASLD’s fiscal year (FY) 2026 appropriations report language on chronic liver disease (CLD). Specifically, the language highlights the burden associated with CLD and the need for a coordinated strategy to advance prevention, screening, and research on CLD across the Department of Health and Human Services (HHS).
AASLD needs your help to get members of the House of Representatives to sign this Dear Colleague letter in support of our report language request. Please act now and ask your Representative to sign this letter to help guide future investments in prevention, detection, and treatment to address the rising burden of CLD.
Please use this template email here and email Lucas Coyle at lcoyle@dc-crd.com for your Representative’s email address.
HHS Undergoes a Major Reorganization: April 11, 2025
On March 27, the Department of Health and Human Services (HHS) unveiled a major reorganization of its structure and agencies. These changes are expected to have significant implications for the agencies and programs prioritized by AASLD, including the National Institutes of Health (NIH), the Centers for Disease Control and Prevention (CDC), the Health Resources and Services Administration (HRSA),and the Food and Drug Administration (FDA).
The Department’s restructuring will reduce the number of HHS divisions from 25 to 12. Its workforce will be cut by approximately 10,000 employees across the department’s various agencies. This reduction follows the departure of nearly 10,000 employees through early retirements and the “Fork in the Road” offer, shrinking the total workforce by 24%, from 82,000 to 62,000 full-time employees. Specifically, the reductions will include 3,500 employees from the FDA – about 19% of the agency’s workforce; 2,400 employees from the CDC – about 18% of its workforce; 1,200 employees from the NIH – about 6% of its workforce; and 300 employees from the Centers for Medicare & Medicaid Services (CMS) – about 4% of its workforce.
At the NIH, the administration is consolidating functions previously carried out by the individual institutes and centers in the Office of the Director. Institutes will no longer have their own policy/legislative affairs, communications, grants management, procurement, travel, ethics, and information technology teams. Most of the institute and center staff who performed these functions were let go as part of the reduction in force. Additionally, five institute and center directors were let go, including Dr. Jeanne Marrazzo who had led the National Institute of Allergy and Infectious Diseases.
NIH plans to consolidate all grant reviews in the Center for Scientific Review (CSR), meaning that institutes and centers will no longer review contracts and training grants or have special emphasis panels. We anticipate this consolidation will occur after the May Council meetings. CSR has never reviewed contracts, which are primarily used to support networks and clinical trials; they will have to acquire the infrastructure to support these reviews, but no update has been provided on if and when this will happen. We expect there will be disruptions to existing and new contracts during the transition. Another complicating factor is that NIH has been directed by the Department of Government Efficiency to cut 35% of its contracts. Please consider sharing your story if your research has been affected by this reorganization, the funding freeze, or other recent changes at NIH.
The changes at the CDC have been particularly disruptive. While the CDC Division of Viral Hepatitis was retained, its viral hepatitis laboratory was eliminated. Members have already reported challenges since its closure and the loss of a laboratory to test these samples. The viral hepatitis laboratory performed molecular sequencing and computational analysis to identify viral hepatitis outbreaks, describe the size and scope, and determine mechanisms of transmission at no cost to those submitting the samples. From 2020-2025, the CDC’s viral hepatitis laboratory provided services to state and local public health authorities for over 52 different viral hepatitis outbreak investigations across the country at no cost. At the time it was shut down, the laboratory was providing response support for a hepatitis C outbreak associated with a pain clinic in Florida. AASLD is working with members of Congress to attempt to restore the laboratory and is asking members who participated in our March 14 Virtual Capitol Hill Day to follow up with Congressional staff on this issue.
Additionally, HRSA will be consolidated into a new Administration for Healthy America (AHA) along with the Office of the Assistant Secretary for Health, the Substance Abuse and Mental Health Administration, the Agency for Toxic Substances and Disease Registry, and the National Institute for Occupational Safety and Health. From the information released to date, it is unclear what the future of these programs will be. Secretary Robert F. Kennedy, Jr. has stated that AHA will more efficiently coordinate chronic care and disease prevention programs as well as harmonize health resources to low-income Americans. There has been no information about the future of HRSA’s Division of Transplantation, which manages the Organ Procurement & Transplantation Network, and how AHA will support this important agency function. AASLD will continue to keep members updated on the future of this program.
In a video posted to X, Secretary Kennedy said HHS is “keenly focused on pairing away excess administrators, while increasing the number of scientists and frontline health providers.” HHS also said that it will ensure that Medicare, Medicaid, and other health services will not be impacted in the restructuring. However, public health experts believe that maintaining services will be a challenge given the complexities of the reorganization.
Following this announcement, Democratic members of Congress have expressed frustration calling on their Republican colleagues to prevent “devastating cuts.” Additionally, House Appropriations Committee Ranking Member Rosa DeLauro (D-CT) issued a statement noting this effort is a direct violation of the full-year appropriations Act that Congress passed in mid-March directing agencies to administer specific programs.
AASLD submitted testimony to the House Labor, Health and Human Services, Education, and Related Agencies Subcommittee on our Fiscal Year 2026 appropriations priorities, which addressed challenges raised by the reorganization.
Virtual Hill Day Report: March 21, 2025
On March 14, AASLD hosted a successful virtual Capitol Hill Day, bringing together 98 AASLD members and patient advocates from across the country to participate in virtual meetings with Congressional offices. Through a series of 103 virtual meetings, representing 38 states, participants advocated for critical health policy issues related to supporting and advancing liver disease research, treatment, and prevention.
Our Asks –
- Support hepatitis C elimination in the United States.
- Support AASLD’s report language requesting the Secretary of the Department of Health and Human Services (HHS) to assemble a report and briefing on existing programs and investments in prevention, screening, and research on chronic liver disease.
- Include at least $51.303 billion for the National Institutes of Health (NIH) in the Labor-HHS appropriations bill for FY 2026.
- Include $150 million for the Centers for Disease Control and Prevention (CDC) Division of Viral Hepatitis in the Labor-HHS appropriations bill for FY 2026.
AASLD’s annual Capitol Hill Day provided a powerful platform for participants to share their expertise, personal experiences, and real-world impact of federal policies. These compelling stories highlighted the urgent need for increased investments in liver disease prevention, treatment, and research. Additionally, participants were able to address how the current threats to federal investment in critical health programs will undermine their work and health of those living with liver disease.
AASLD remains committed to building on this momentum by continuing to advocate for policies that to improve the lives of people impacted by liver disease. We are grateful to our members for being a strong voice for the liver disease community and for their dedication to advancing meaningful policy change. Please watch for opportunities to support AASLD’s advocacy efforts.
Dr. Jay Bhattacharya, Trump’s Pick to Head NIH, Goes Before Senate HELP Committee: March 7, 2025
On Wednesday morning, Dr. Jay Bhattacharya, a Stanford health economist who gained national attention for his criticism of the COVID lockdowns, went before the Senate Health, Education, Labor, and Pensions (HELP) Committee to articulate his vision for the National Institutes of Health (NIH). President Donald Trump’s nominee to lead the $48 billion agency opened his remarks by sharing his experience as a NIH-funded researcher on aging populations, chronic disease, and obesity and recognizing the leadership role the agency plays in biomedical research.
Bhattacharya articulated five goals to advance the agency’s mission—to support scientific discovery to enhance health and lengthen life:
- Focus research to solve the country’s chronic disease crisis;
- Ensure that NIH-supported research is replicable, reproducible, and generalizable;
- Establish a culture of respect for free speech in science and scientific dissent at the NIH;
- Recommit the agency to its mission to fund the most innovative biomedical research agenda possible; and
- Vigorously regulate risky research that has the possibility to cause a pandemic.
Repeatedly, Bhattacharya addressed the public’s distrust in science and how his goals, particularly ensuring that research is replicable, reproducible, and generalizable, would address these concerns. During intense questioning by HELP Committee Chair Bill Cassidy (R-LA), Bhattacharya said that he does not believe vaccines cause autism, but he would not agree that additional research on the link between vaccines and autism wasn’t required; his support for additional research was consistent with the position that Department of Health and Human Services Secretary Robert F. Kennedy, Jr. took before the same committee. Senator Susan Collins (R-ME) used her time to inquire about how Bhattacharya will address the administration’s policy to cap facilities and administrative costs, indicating that a one size fits all approach does not make sense. In response, Bhattacharya pledged to work with her on that issue.
Democrats on the committee focused most of their questions on the Trump administration’s recent policy changes at NIH: the halting of study sections and some research funding as well as additional questions on the facilities and administrative costs policy. He did indicate that he would restart the study sections that have been paused. While Bhattacharya promised to ensure that researchers have the resources they need to conduct their work, he would not commit to reversing the administration’s policies. He did say that he did not believe that ideology should determine the direction of science. Bhattacharya did commit to preserving diversity in clinical trials despite President Trump’s Executive Order halting federal DEI programs.
AASLD submitted questions on topics ranging from indirect costs to alcohol-associated liver disease and liver transplantation for the record. While the questions specific to liver disease were not asked directly at the hearing, Bhattacharya will be responding in writing to them. AASLD is closely following cuts to biomedical research funding and changes in related policy and is taking every opportunity to advocate to protect this research.
Monitoring New Administration Policies and Executive Orders: February 15, 2025
AASLD continues to lead advocacy efforts on behalf of its members in Washington as the new administration and Congress establish their priorities for the year. We will advocate to protect biomedical research funding and patients access to high-quality care for liver diseases.
NIH Cap on Facility and Administrative Costs
On the evening of February 7, the NIH released guidance setting a 15% ceiling for facilities and administrative costs—also known as indirect costs—stating, “For any new grant issued, and for all existing grants to institutions of higher education (IHEs) retroactive to the date of issuance of this Supplemental Guidance, award recipients are subject to a 15% indirect cost rate.” Senator Patty Murray (D-WA), Vice Chair of the Senate Appropriations Committee, immediately released a statement asserting that this executive action is illegal because the Labor-HHS appropriations bill prohibits modifications to the NIH’s indirect costs.
Last week, a federal judge issued a nationwide temporary restraining order prohibiting the implementation of this policy. In the order, the judge wrote that the defendants cannot take “any steps to implement, apply, or enforce the [policy] … in any form with respect to institutions nationwide until further order is issued by this Court.” A hearing has been set for February 21 for both the cases filed challenging NIH’s cap on these facilities and administration costs. We will be watching this issue very closely.
Update on Data on Federal Websites
In our last update, we shared that the Trump administration has removed certain information and data sets from federal websites to ensure it is compliant with the Executive Order ending programs related to DEI. On February 11, a federal judge issued a temporary restraining order was granted and required federal health agencies to restore the websites and data sets that had been taken down. Much of this information has come back online but it is not clear what, if any, information has been altered or is still missing.
During our recent conversations with congressional staff, they highlighted how important it is for them to hear how these policies are affecting their constituents. To do this, AASLD is asking you to share your story. Please consider supporting our advocacy efforts to support biomedical research and patients’ access to new and existing therapies by completing this form.
Monitoring New Administration Policies and Executive Orders: February 7, 2025
On January 21, the Acting Secretary of the Department of Health and Human Services (HHS) Dorothy Fink issued a memo requiring health agencies to pause their public communications through February 1. This pause applied to the publication of documents, regulations, guidances, grant announcements, website updates, press releases, and social media. Additionally, the staff at HHS agencies were prohibited from attending public meetings, including speaking engagements, and from work-related travel. This policy’s implementation was especially disruptive at the National Institutes of Health (NIH) where study section and institute and center council meetings were suspended.
As of February 5, this prohibition on public communications remains partially in effect indefinitely as some communications and activities are beginning to resume across HHS. At the NIH, study sections and some institute and center advisory committee meetings are resuming, although the advisory councils are only meeting in closed sessions. Acting NIH Director Matthew Memoli has conveyed that NIH scientists may resume travel for site visits and collaborative research. However, agency researchers are still prohibited from traveling for scientific meetings or participating in speaking engagements.
Additionally, the NIH, and other HHS and federal agencies, have been directed to review programs and grant awards to ensure that they are consistent with President Trump’s Executive Orders. Specifically, agencies have been instructed to halt those related to Diversity, Equity, and Inclusion (DEI) to be compliant with the administration’s policy. Some federal websites, including public health information data sets, have been removed. It is our understanding that some of this information may come back online if it is found to be consistent with the Executive Orders. We will continue to monitor this situation.
AASLD will convene a virtual Hill Day on Friday, March 14, to engage with Congress on these issues and its 2025 public policy agenda. This event is open to all AASLD members, and we encourage your participation and advocacy. For more information, contact Mike Providence, Director of Policy & Planning, at mprovidence@aasld.org.
HHS Releases 2023 Viral Hepatitis Report
- On August 14, the U.S. Department of Health and Human Services (HHS) Office of Infectious Disease and HIV/AIDS Policy released updates on viral hepatitis. While much has been achieved, much more needs to be done. Here is a progress report on the current Viral Hepatitis Strategic Plan.
NIH Reform & Appropriations
- Recently, the House Appropriations Committee released its report accompanying its FY25 Labor-Health and Human Services bill. A link to the bill text released a few weeks ago can be found here.
In this report, the House Appropriations Committee adopted a proposal from Energy and Commerce Committee Chair Cathy McMorris Rodgers to restructure the NIH without any public hearings on the topic. AASLD signed a letter expressing concern about the restructuring of the NIH, including reducing the number of agencies from 27 to 15, and consolidation of the NIDDK into a larger institute. The letter went to the House Appropriations Committee leadership on 7/9/2024 with 223 organizations having signed. AASLD is gravely concerned that NIH’s investment in liver disease research will suffer substantially if this policy is enacted as proposed and will be submitting comments by the deadline of August 16. Members are encouraged to send their concerns to Chair McMorris Rodgers by highlighting the impact the policy would have on their research.
HCV Elimination
- In June, the Congressional Budget Office (CBO) released a report examining policies that would increase treatment of hepatitis C in the Medicaid population and requested research and data related to HCV prevalence and elimination strategies. AASLD received expert feedback from our special interest groups and coalition partners in providing feedback to the CBO. AASLD is hopeful that legislation to eliminate hepatitis C, stemming from the White House HCV Elimination Plan, will be introduced in 2024.
CMS News
- The Centers for Medicare & Medicaid Services (CMS) just released the CY 2025 Medicare Physician Fee Schedule (MPFS) proposed rule. Notably, payment rates under the MPFS are proposed to be reduced by 2.80% and the proposed conversion factor is $32.36, a decrease of $0.93, in CY 2025.
Here are the rule, press release, and fact sheet for your review.
- CMS has included expansion of access to hepatitis B vaccines in Medicare Part B as part of its 2025 MPFS proposed rule. Specifically, CMS is proposing to expand the definition of those at intermediate risk of contracting hepatitis B – to include those who did not complete their hepatitis B immunization series and those whose vaccination status is unknow – and proposing to eliminate the requirement that a doctor's order be needed for the administration of a hepatitis B vaccine. Therefore, Medicare Part B recipients would be able to receive a vaccination at a pharmacy, a senior center, or another venue that would be more convenient. Hepatitis B vaccines would also be added to the Medicare Part B "mass immunizer program" which allows for broad access to vaccines. The proposed rule also specifically mentions that this change would assist the nation's efforts to eliminate viral hepatitis.
AASLD has met with CMS and key Hill offices as well as signed a coalition letter advocating for this change.
CMS is accepting public comments through September 9, and we will need comments from as many community members and other stakeholders as possible to make sure this rule is finalized and that more individuals will have access to hepatitis B vaccinations.
Other Advocacy Updates
AASLD provided feedback via comment or sign-on letter for the following legislation:
- The Pay PCPs Act: expressed our support for the creation of a technical advisory committee, which would adopt an evidence-based approach to valuing evaluation and management and other cognitive services.
- Expedited Consideration of NCD for HCV Screening in Non-Primary Care Settings
- Preserving funding for the AHRQ (Agency for Healthcare Research and Quality)
- Opposing reductions to non-defense discretionary appropriations in fiscal year 2025
For more information, contact Erika Miller.
Previous Efforts
AASLD is committed to educating Congress and key federal officials on the growing burden of liver disease, to inform policies that support research and treatment of hepatic disorders. Along these lines, the Public Policy Committee has recently:
Increasing Hepatitis C treatment in the Medicaid population (6/17/2024)
On June 14, the Congressional Budget Office (CBO) released a blog post and report on increasing hepatitis C treatment in the Medicaid population, which shows that there will be savings if more Medicaid beneficiaries are treated. This is not a score, and the CBO called for more research in the following three areas:
- The effectiveness of outreach and other efforts in increasing testing, treatment initiation, and adherence among targeted populations (which would also depend on the specific populations included in the program);
- The costs of treating hepatitis C with DAAs; and
- The costs of treating complications from hepatitis C if the disease is untreated.
Besides these three areas, the CBO is also interested in updated data on the costs associated with untreated hepatitis C and the longer-term effects of expanded hepatitis C treatment. The CBO did not provide a deadline for submitting this information and other comments. AASLD will be working to compile information that can be shared with CBO in response and will be convening the HCV elimination coalition to solicit feedback from the group.
For more information, contact Erika Miller.
HCV Plan Rural Health Impact
On behalf of the 70 undersigned organizations representing patients, providers, and public health organizations, we urge Congress to adopt legislation to eliminate hepatitis C and deliver better outcomes for hepatitis patients living in rural communities. These communities are disproportionately impacted by hepatitis C, seeing infection rates that are often estimated to be twice as high as those in urban areas. Fortunately, hepatitis C is curable with an 8-to-12-week oral therapy that is highly tolerated and over 95 percent effective. This epidemic does not have to continue in our most vulnerable communities, and passing legislation to eliminate hepatitis C will ensure that all Americans can know their hepatitis status and be connected to a low-cost cure. See the letter here.
Capitol Hill 2024 in Review
On April 4, AASLD welcomed 51 advocates, including AASLD members, patient advocates, and society staff, in support of advancing priorities related to the prevention and treatment of liver disease. Advocates joined 54 meaningful conversations with members of Congress and their staff to discuss AASLD’s top priorities. Specifically, participants urged members to (1) support authorizing legislation to implement the White House’s national plan to eliminate hepatitis C in the United States in five years; and (2) support for the development of a Department of Health and Human Services-wide strategic plan to combat all liver diseases. AASLD’s fact sheets on both requests can be found here.
Advocates met with the staff of members of Congress who are on committees relevant to these legislative requests. Additionally, they shared personal perspectives regarding their experience living with or caring for patients with liver disease. They also shared key liver disease statistics and findings relevant to their state and district. AASLD members and patient advocates made a lasting impact through these conversations, and we look forward to staying engaged with members of Congress and continuing to reinforce the need to prioritize the prevention, treatment, and study of liver diseases.
Quick stats:
- 51 advocates from 16 states and Washington, D.C.
- 54 meetings – 32 in the Senate and 22 in the House
- 7 meetings with Committee Leadership
- 27 meetings with members on Committees relevant to AASLD requests
If you have any questions regarding AASLD’s advocacy, please contact Erika Miller.
Friends of NIDDK
As a member of the Friends of the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK), AASLD joined with 30 other patient, provider, and research organizations to urge Congress to finalize the fiscal year (FY) 2023 omnibus funding bill with at least $47.5 billion for the base budget of the National Institutes of Health (NIH) and a comparable increase of at least $2.485 for the NIDDK to ensure the US continues to lead the world in providing new and better cures, diagnostics, and treatments while protecting all patients and the research enterprise. See the letter here.
Combatting Liver Disease: Eliminating Hepatitis C
On Wednesday, March 15, 2023, AASLD convened for the third installment in the Combatting Liver Disease briefing series focusing on the elimination of hepatitis C. The briefing included a robust discussion on the public health burden of hepatitis C and the Biden Administration's plans to develop a national hepatitis C elimination program. AASLD was fortunate to be joined by a panel of experts in the field, including:
- Dr. Francis Collins, Special Project Advisor to President Joe Biden and Former Director of the National Institutes of Health
- Dr. Norah Terrault, AASLD President, and Professor of Medicine and Chief of Gastroenterology and Liver Diseases at Keck Medical Center of the University of Southern California
- Dr. Julius Wilder, AASLD Public Policy Committee Co-Chair and Assistant Professor of Medicine at Duke University School of Medicine
- Ryan Clary, Hepatitis Advocate and Non-Profit Consultant
If you were unable to join us live, we recommend watching the recording below to learn more about the three main components of the White House's elimination plan: details on the disease progression and burden of hepatitis C if no action is taken, perspectives of a long time hepatitis C advocate who has been personally impacted by the disease, and the significant actions AASLD is taking to eliminate hepatitis C.
RECORDING LINK: HERE
Combatting Liver Disease: Liver Cancer
On December 6, 2022, AASLD held its second briefing in the Combatting Liver Disease series focused on liver cancer – the third leading cause of cancer death in 2020 – and important public policy implications. The briefing was moderated by Dr. Steven Lidofsky, Chair of AASLD's Public Policy Committee, and featured remarks from several distinguished leaders in the field and insights from the patient perspective.
Specifically, panelists included:
- Dr. Doug Lowy, National Cancer Institute
- Dr. Liz Pomfret, University of Colorado
- Dr. Lewis Roberts, Mayo Clinic
- Tony Villiotti, Founder of NASH kNOWledge, and a liver cancer and liver transplant survivor
By watching the briefing, you will hear an overview of the issue, a discussion of what it’s like to live with liver cancer, and both the public health practitioner and medical research perspective on policies that can help combat liver cancer.
RECORDING LINK: HERE
Combatting Liver Disease: Alcohol-Associated Liver Disease
On Tuesday, September 20, 2022, AASLD sponsored the first Congressional briefing in their Combatting Liver Disease series for policy makers focusing on alcohol-associated liver disease. Moderated by AASLD Governing Board President Dr. Laurie DeLeve, speakers included:
- Dr. George Koob, Director of the National Institute on Alcohol Abuse and Alcoholism
- Dr. Vijay Shah, Mayo Clinic (AASLD Board Treasurer)
- Dr. Jessica Mellinger, University of Michigan (AASLD Member)
- Brian Bourgault, a person living with ALD
Speakers briefed the audience on changes to the ALD population, the challenges of securing funding for ALD-related research, and the perspective of someone who lives with a liver transplant due to ALD. Additional discussion focused on the future of digital health and AI in clinical trials and managing patient care for people who are at risk or develop ALD.
RECORDING LINK: HERE
Advocacy for Telehealth
Telehealth flexibilities have improved access to hepatology specialists during the COVID-19 pandemic and will remain an important tool for delivering comprehensive care to patients with liver disease. In March 2022, Congress approved a 151-day extension of certain telehealth flexibilities, including the elimination of the originating site and geographic restrictions and coverage of audio-only services, once the public health emergency concludes. AASLD will continue to advocate for these flexibilities as well as continued payment parity between in-person and telehealth services.
Telehealth Stakeholder Letter to the Senate
AASLD Weighs in on ARPA-H
President Biden proposed the creation of a new research accelerator called the Advanced Research Projects Agency for Health (ARPA-H) in his Fiscal Year 2022 budget request. Congress appropriated the first $1 billion for the new entity in its FY 2022 omnibus spending package. AASLD supports the new entity in concept and believes it has the potential to advance our understanding of liver disease. However, the society is concerned that funding for the new entity will supplant, not supplement, money for the National Institutes of Health (NIH). To prevent the lines between the two entities from blurring, AASLD is advocating for ARPA-H to be placed outside NIH.
Policy Agenda
Each year, the Public Policy Committee develops a policy agenda, which is then approved by the Governing Board, to guide our annual advocacy activities. While the agenda serves as a framework for implementing strategic initiatives, it is recognized that AASLD is prepared to respond dynamically to unforeseen opportunities or threats that may reshape its actions.
COVID-19 Checklists and Tools to Accelerate Relief for State Medicaid & CHIP Programs
- 1115 Waiver Opportunity and Application Checklist
- 1135 Waiver Checklist
- 1915(c) Appendix K Template
- Medicaid Disaster State Plan Amendment Template
On Monday, March 30, 2020, CMS released an interim final rule and blanket waivers to provide additional flexibilities to providers during the COVID-19 emergency. AASLD’s Government Relations Consultant CRD Associates prepared this summary [PDF].
CMS released additional waivers on April 9. All of the waivers can be found online [PDF].